The Irreversible Transformation of the Molecular Structure of Humic Acid during pH Change and Its Effects on the Formation of Disinfection By-Products
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
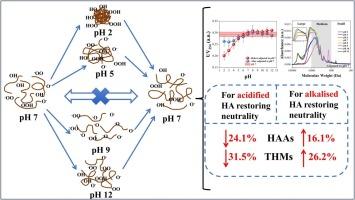
Humic acid (HA) is an important component of natural organic matter, and understanding the nature and environmental behavior of HA is essential for advancing water treatment technologies and environmental remediation strategies. This study investigated the structural differences of HA at various pH values and whether the structure is reversible (whether the structure is similar when HA at different pH values is adjusted back to neutral compared to the original pH 7) by optical characteristics, hydrodynamic volume, fluorescence, infrared and circular dichroism spectroscopy. After adjustment back to neutral, from prior exposure to different pH values (2-12), the results showed an irreversible behavior of HA. For acidified HA restoring neutrality, the TOC and UV254 values decreased by 12.2% and 11.2%, respectively, and the formation of haloacetic acids (HAAs) and trihalomethanes (THMs) decreased by 24.1% and 31.5%, respectively. These changes were attributed to the protonation of oxygenated groups, the weakening of hydrogen bonding, resulting in the formation of aggregates by HA molecules and hydrophilic and hydrophobic structural changes. For alkalized HA restoring neutrality, the TOC increased by 10.7%, and the formation of HAAs and THMs increased by 16.1% and 26.2%, respectively. These changes were attributed to the increase of electronegativity following deprotonation of HA functional groups, molecular swelling caused by increased molecular repulsion, and twisting of the secondary structure. This study provides new insights regarding the effect of changes in pH conditions on the structure and reactivity of HA, which are important for future approaches to the removal of HA and management of disinfection by-products (DBPs) formation in water treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


