Surface-Functionalized Polydimethylsiloxane Sponges for Facile and Selective Recovery of Molybdenum from Aqueous/Acidic Solutions
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
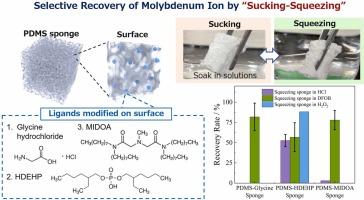
The present study fabricated two types of surface-functionalized polydimethylsiloxane sponges (amine-functionalized and extractant-impregnated), and investigated their applicability as a novel separation-recovery method for molybdenum ion (Mo(VI)) in aqueous and HNO3 solutions. An amine-functionalized polydimethylsiloxane (PDMS) sponge was prepared using glycine hydrochloride as a functional ligand, called the Glycine-PDMS sponge, and bis(2-ethylhexyl) phosphate (HDEHP) and 2,2'-(Methylimino)bis(N,N-di-n-octylacetamide) (MIDOA) were used as extractants for the extractant-impregnated sponges, called the HDEHP-PDMS and MIDOA-PDMS sponges, respectively. The adsorption and desorption of Mo(VI) were demonstrated by facile soaking and squeezing of the sponges, which is unavailable in conventional adsorbents. Each PDMS sponge enabled selective Mo adsorption in aqueous and acidic solutions, and had different ligand and HNO3 concentration dependence of the Mo(VI) adsorption capacities. The pseudo-second-order kinetic model was found to be applicable for the Mo(VI) adsorption process on the sponge surfaces. A regression analysis of the isothermal adsorption curves for Mo ions clarified that the adsorption of Mo ions onto all PDMS sponges occurs spontaneously, and that Mo(VI) adsorption mechanism is different depending on sponges; chemisorption for extractant-impregnated sponges and physisorption for an amine-functionalized, respectively. Furthermore, the squeezing of the Mo ion adsorbed PDMS sponges allowed the rapid desorption and recovery of Mo ions into eluent solutions such as deferoxamine B (DFOB) and H2O2 within a few minutes. These results prove that the novel PDMS sponge approach, enabling facile chemical-mechanical adsorption and desorption control of target elements, has great potential in various fields involving the environment, medicine, energy, and so on.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


