Low-Temperature Highly Efficient Catalytic Removal of Odorous Carbonyl Sulfide by Facile Regulating CeO2 Morphologies
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
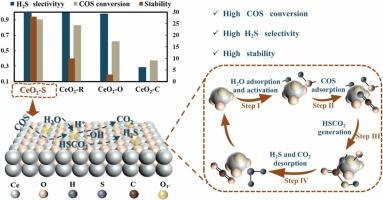
Unraveling the water activation is essential in the catalytic hydrolysis of organic sulfur compounds, yet its intrinsic mechanism of the water-promoting effect is still unclear. In this work, we describe novel findings of oxygen vacancy (VO) engineering by facile regulating CeO2 nanocatalysts with different shapes (rod, octahedral, sphere and cube) for COS hydrolysis at lower temperature, aiming at understanding the structural origin of the excellent catalytic hydrolysis activity. Unexpectedly, among CeO2 catalysts with different morphologies, spherical CeO2 (CeO2-S) catalysts can achieve completely conversion of COS at 60 ℃ and maintain 30 hours of non-deactivation, which is a significant improvement in catalytic activity and reaction temperature compared to previously reported catalysts. Through various characterizations and results analysis, it is obvious to see that the more spontaneous formation VO on CeO2-S catalysts synergistically induced the water activation and dissociation thus result in the generation of more surface active hydroxyl groups (-OH), which contributes to the enhanced performance of COS catalytic hydrolysis at lower temperature. The promoting effect of catalyst morphology changes on COS hydrolysis were furthering analyzed using in situ DRIFTS and DFT calculations, and revealed that the exposed (111) crystal plane of CeO2 exhibits the strongest adsorption capacity for COS. Notably, CeO2-S also exhibited good catalytic performance and stability towards to other typical organic sulfur compounds (COS and CS2), which is beneficial for the wide application at complex operating conditions. This study provides new insights for designing OH-rich CeO2 catalysts to remove single as well as multi-component organic sulfur compounds for different applications at lower temperatures.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


