Effect of Brief Electrical Stimulation on Cell Biomechanics in Hereditary Sensory Neuropathy
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
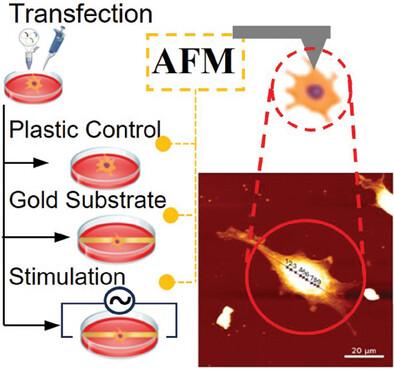
SH-SY5Y neuroblastoma cells are widely used to model neurodegenerative disorders like Alzheimer's, Parkinson's, Huntington's, and Hereditary Sensory Neuropathy type 1A (HSN-1A), a peripheral nerve condition causing axon degeneration and sensory loss. A cell model of HSN-1A is developed by overexpressing wild-type and mutant SPTLC1 genes (C133W, C133Y, V144D). Cells are cultured on plastic and gold substrates, with brief electrical stimulation applied to the gold-grown cells. Atomic force microscopy (AFM) is used to measure Young's modulus, indentation, and energy dissipation. Finite Element Method and non-linear modeling validate the results. In the absence of stimulation, mutant cells show lower stiffness compared to non-transfected cells, indicating a direct biomechanical impact of the mutations. Brief electrical stimulation significantly increases the stiffness of mutant cells, particularly in C133W (99%), C133Y (100%), and V144D (111%) variants, despite the mutations. Energy dissipation of stimulated V144D cells decreases to levels comparable to untreated non-transfected cells. The simulations support the AFM measurements, demonstrating that brief electrical stimulation can partially reverse the biomechanical effects of gene mutations.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


