Molecular signature of primate astrocytes reveals pathways and regulatory changes contributing to human brain evolution
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Astrocytes contribute to the development and regulation of the higher-level functions of the brain, the critical targets of evolution. However, how astrocytes evolve in primates is unsettled. Here, we obtain human, chimpanzee, and macaque induced pluripotent stem-cell-derived astrocytes (iAstrocytes). Human iAstrocytes are bigger and more complex than the non-human primate iAstrocytes. We identify new loci contributing to the increased human astrocyte. We show that genes and pathways implicated in long-range intercellular signaling are activated in the human iAstrocytes and partake in controlling iAstrocyte complexity. Genes downregulated in human iAstrocytes frequently relate to neurological disorders and were decreased in adult brain samples. Through regulome analysis and machine learning, we uncover that functional activation of enhancers coincides with a previously unappreciated, pervasive gain of “stripe” transcription factor binding sites. Altogether, we reveal the transcriptomic signature of primate astrocyte evolution and a mechanism driving the acquisition of the regulatory potential of enhancers.
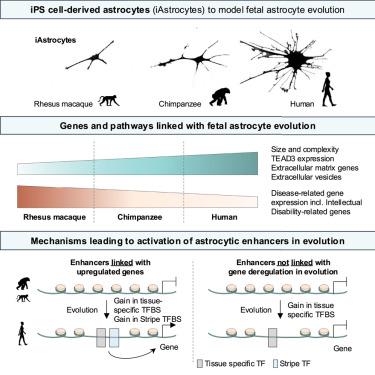
灵长类星形胶质细胞的分子特征揭示了促进人类大脑进化的途径和调节变化
星形胶质细胞有助于大脑高级功能的发育和调节,是进化的关键目标。然而,灵长类动物的星形胶质细胞是如何进化的尚不清楚。在这里,我们获得了人类、黑猩猩和猕猴诱导的多能干细胞来源的星形胶质细胞(iAstrocytes)。人类的iAstrocytes比非人灵长类动物的iAstrocytes更大更复杂。我们发现了有助于增加人类星形胶质细胞的新位点。我们发现,参与长距离细胞间信号传导的基因和途径在人类星形胶质细胞中被激活,并参与控制星形胶质细胞的复杂性。人类星形胶质细胞中下调的基因通常与神经系统疾病有关,并且在成人脑样本中减少。通过规则组分析和机器学习,我们发现增强子的功能激活与以前未被认识到的“条纹”转录因子结合位点的普遍增加相一致。总之,我们揭示了灵长类星形胶质细胞进化的转录组学特征和驱动增强子获得调控潜力的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


