Deciphering the Role of PEGylation on the Lipid Nanoparticle-Mediated mRNA Delivery to the Liver
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
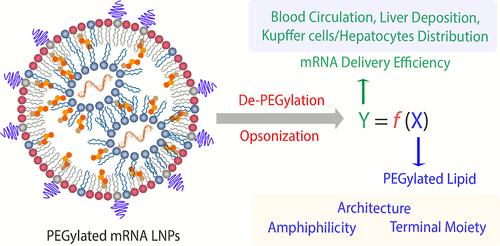
Organ- and cell-specific delivery of mRNA via modular lipid nanoparticles (LNPs) is promising in treating various diseases, but targeted cargo delivery is still very challenging. Most previous work focuses on screening ionizable and helper lipids to address the above issues. Here, we report the multifacial role of PEGylated lipids in manipulating LNP-mediated delivery of mRNA to the liver. We employed the typical excipients in LNP products, including DLin-MC3-DMA, DPSC, and cholesterol. Five types of PEGylated lipids were selected, and their molar ratio was fixed at 1.5% with a constant PEG molecular weight of 2000 Da. The architecture of steric lipids dramatically affected the in vitro gene transfection, in vivo blood clearance, liver deposition, and targeting of specific cells, all of which were closely linked to the de-PEGylation rate. The fast de-PEGylation resulted in short blood circulation and high accumulation in the liver. However, the ultrafast de-PEGylation enabled the deposition of more LNPs in Kupffer cells other than hepatocytes. Surprisingly, simply changing the terminal groups of PEGylated lipids from methoxyl to carboxyl or amine could dramatically increase the liver delivery of LNPs, which might be associated with the accelerated de-PEGylation rate and enhanced LNP–cell interaction. The current work highlights the importance of manipulating steric lipids in promoting mRNA delivery, offering an alternative approach for formulating and optimizing mRNA LNPs.
解密 PEG 化对脂质纳米颗粒介导的 mRNA 向肝脏递送的作用
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


