The lactate receptor HCAR1 drives the recruitment of immunosuppressive PMN-MDSCs in colorectal cancer
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
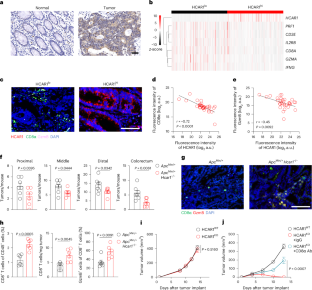
Most patients with colorectal cancer do not achieve durable clinical benefits from immunotherapy, underscoring the existence of alternative immunosuppressive mechanisms. Here we found that activation of the lactate receptor HCAR1 signaling pathway induced the expression of chemokines CCL2 and CCL7 in colorectal tumor cells, leading to the recruitment of immunosuppressive CCR2+ polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) to the tumor microenvironment. Ablation of Hcar1 in mice with colorectal tumors significantly decreased the abundance of tumor-infiltrating CCR2+ PMN-MDSCs, enhanced the activation of CD8+ T cells and, consequently, reduced tumor burden. We detected immunosuppressive CCR2+ PMN-MDSCs in tumor specimens from individuals with colorectal and other cancers. The US Food and Drug Administration-approved drug reserpine suppressed lactate-mediated HCAR1 activation, impaired the recruitment of CCR2+ PMN-MDSCs, boosted CD8+ T cell-dependent antitumor immunity and sensitized immunotherapy-resistant tumors to programmed cell death protein 1 antibody therapy in mice with colorectal tumors. Altogether, we described HCAR1-driven recruitment of CCR2+ PMN-MDSCs as a mechanism of immunosuppression. Lu and colleagues show that signaling through the lactate receptor HCAR1 in colorectal tumor cells promotes the recruitment of immunosuppressive CCR2+ PMN-MDSCs and that inhibition of HCAR1 by reserpine augments the CD8+ T cell-mediated antitumor immune response.

乳酸受体HCAR1驱动免疫抑制PMN-MDSCs在结直肠癌中的募集
大多数结直肠癌患者不能从免疫治疗中获得持久的临床益处,这强调了存在其他免疫抑制机制。本研究发现,乳酸受体HCAR1信号通路的激活可诱导趋化因子CCL2和CCL7在结直肠肿瘤细胞中的表达,导致免疫抑制性CCR2+多态核髓源性抑制细胞(PMN-MDSCs)向肿瘤微环境募集。在结直肠肿瘤小鼠中消融Hcar1可显著降低肿瘤浸润性CCR2+ PMN-MDSCs的丰度,增强CD8+ T细胞的活化,从而减轻肿瘤负荷。我们在结直肠癌和其他癌症患者的肿瘤标本中检测到免疫抑制CCR2+ PMN-MDSCs。美国食品和药物管理局批准的药物利血平抑制乳酸介导的HCAR1激活,损害CCR2+ PMN-MDSCs的募集,增强CD8+ T细胞依赖性抗肿瘤免疫,并使免疫治疗耐药肿瘤对程序性细胞死亡蛋白1抗体治疗增敏。总之,我们描述了hcar1驱动的CCR2+ PMN-MDSCs募集是一种免疫抑制机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


