Quantifying cell divisions along evolutionary lineages in cancer
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
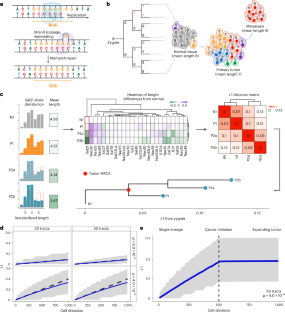
Cell division drives somatic evolution but is challenging to quantify. We developed a framework to count cell divisions with DNA replication-related mutations in polyguanine homopolymers. Analyzing 505 samples from 37 patients, we studied the milestones of colorectal cancer evolution. Primary tumors diversify at ~250 divisions from the founder cell, while distant metastasis divergence occurs significantly later, at ~500 divisions. Notably, distant but not lymph node metastases originate from primary tumor regions that have undergone surplus divisions, tying subclonal expansion to metastatic capacity. Then, we analyzed a cohort of 73 multifocal lung cancers and showed that the cell division burden of the tumors’ common ancestor distinguishes independent primary tumors from intrapulmonary metastases and correlates with patient survival. In lung cancer too, metastatic capacity is tied to more extensive proliferation. The cell division history of human cancers is easily accessible using our simple framework and contains valuable biological and clinical information. This work presents a framework for estimating cell division numbers using DNA replication-associated polyguanine tract mutations, with applications for understanding tumor natural histories and origins.

定量癌症细胞沿进化谱系的分裂
细胞分裂驱动体细胞进化,但很难量化。我们开发了一个框架来计算细胞分裂与DNA复制相关突变的多鸟嘌呤均聚物。我们分析了37例患者的505份样本,研究了结直肠癌演变的里程碑。原发肿瘤在创始细胞分化约250次时发生分化,而远端转移分化发生较晚,约500次。值得注意的是,远端而非淋巴结转移起源于原发肿瘤区域,这些肿瘤区域经历了多余的分裂,将亚克隆扩张与转移能力联系起来。然后,我们分析了73例多灶性肺癌,发现肿瘤共同祖先的细胞分裂负担区分了独立原发肿瘤和肺内转移瘤,并与患者生存相关。肺癌的转移能力也与更广泛的扩散有关。人类癌症的细胞分裂历史很容易使用我们的简单框架,并包含有价值的生物学和临床信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


