Insights into the Redox Chemistry and Structural Evolution of a P2-Type Cathode Material in Sodium-Ion Batteries
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
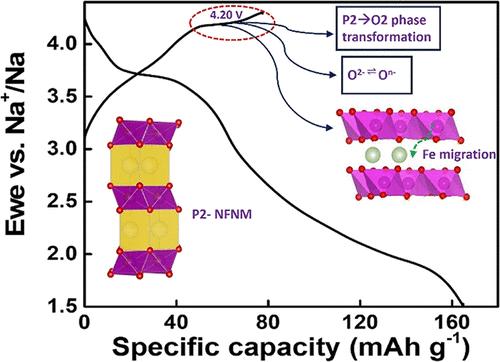
In view of the tunable composition and higher theoretical capacity, layered transition metal oxide cathode materials (NaxTMO2, TM: transition metal) have attained substantial attention. In this work, a P2-type layered metal oxide with the nominal composition Na0.67Fe0.20Ni0.15Mn0.65O2 (NFNM) was synthesized via a sol–gel method; electrochemical performance and the operating mechanism of the electrode material in half-cells were investigated. The material delivered an initial discharge capacity of 166 mA h g–1 where the capacity retention after 50 cycles is 65% when cycled in the voltage range 1.50–4.30 V at a C-rate of C/20. At 1C, the capacity delivered by the material was 110 mA h g–1 and the capacity retention noted after 80 cycles was 80%. A combination of in operando synchrotron diffraction and X-ray absorption spectroscopy (XAS) elucidates the electrochemical mechanism in a Na/NFNM half-cell. The structural evolution of the electrode material was analyzed using in operando XRD from which the evidence of reversible P2-Z phase transformations was obtained. Investigation of the charge-compensation mechanism and local structure changes in the electrode material during cycling were carried out via the XAS technique which revealed the coupled Fe migration, anionic activity, and phase transformations.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


