Engineering Protein-Based Lipid-Binding Nanovesicles via Catechol-Amine-Derived Coacervation with Their Underlying Interfacial Mechanisms
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The development of nonphospholipid nanovesicles has garnered tremendous attention as a viable alternative to traditional liposomal nanovesicles. Protein/peptide-based nanovesicles have demonstrated their potential to reduce immunogenicity while enhancing bioactivity. However, a fundamental understanding of how proteinaceous vesicles interact with lipids and cell membranes remains elusive. In this study, we engineered a series of protamine-based nonphospholipid nanovesicles by modulating intramolecular catechol–amine interactions. By grafting trihydroxybenzene (GA) and catechol (CA) groups onto the protamine (Prot), a salt-triggered coacervation was observed in an alkaline environment with the size of as-prepared vesicles ranging from 200 to 1200 nm. The bonding affinity to lipid interfaces followed the order of Prot-CA-Fe3+(25 μM) > Prot-CA-Fe3+(10 μM) > Prot-CA > original Prot with the underlying nanomechanics investigated by the lipid bubble force measurement. Direct quantification of interactions between the nanovesicles and living human gingival fibroblasts was performed by using surface charge difference mapping. Introducing trace amounts of Fe3+ (at 10 and 25 μM) enhanced vesicle–lipid interactions via the synergy of catechol–amine interactions and Fe3+-induced complexation. This work provides improved valuable insights into the interactions between nanovesicles and cell membranes, offering an energetic paradigm for modulating cell-target delivery processes via intramolecular short-range interactions.
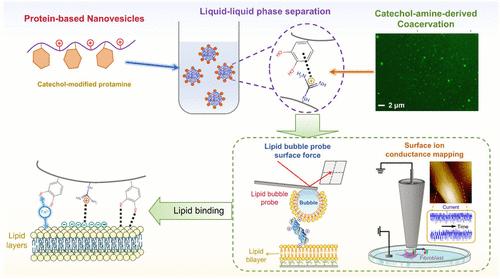
通过儿茶酚--氨产生的共凝作用及其潜在的界面机制设计蛋白型脂质结合纳米囊泡
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


