NLRP3-mediated glutaminolysis controls microglial phagocytosis to promote Alzheimer’s disease progression
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Activation of the NLRP3 inflammasome has been implicated in the pathogenesis of Alzheimer’s disease (AD) via the release of IL-1β and ASC specks. However, whether NLRP3 is involved in pathways beyond this remained unknown. Here, we found that Aβ deposition in vivo directly triggered NLRP3 activation in APP/PS1 mice, which model many features of AD. Loss of NLRP3 increased glutamine- and glutamate-related metabolism and increased expression of microglial Slc1a3, which was associated with enhanced mitochondrial and metabolic activity. The generation of α-ketoglutarate during this process impacted cellular function, including increased clearance of Aβ peptides as well as epigenetic and gene transcription changes. This pathway was conserved between murine and human cells. Critically, we could mimic this effect pharmacologically using NLRP3-specific inhibitors, but only with chronic NLRP3 inhibition. Together, these data demonstrate an additional role for NLRP3, where it can modulate mitochondrial and metabolic function, with important downstream consequences for the progression of AD.
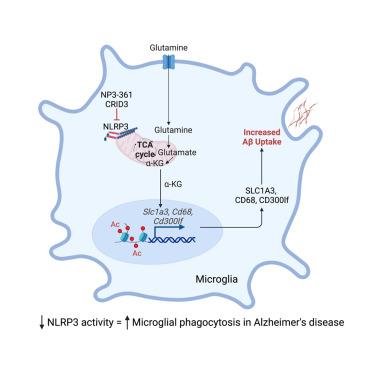
nlrp3介导的谷氨酰胺溶解控制小胶质细胞吞噬促进阿尔茨海默病进展
NLRP3炎性小体的激活通过IL-1β和ASC斑点的释放与阿尔茨海默病(AD)的发病机制有关。然而,NLRP3是否参与其他途径仍然未知。本研究发现,体内Aβ沉积直接触发APP/PS1小鼠NLRP3的激活,从而模拟了AD的许多特征。NLRP3的缺失增加了谷氨酰胺和谷氨酸相关的代谢,增加了小胶质细胞Slc1a3的表达,这与线粒体和代谢活性的增强有关。在此过程中α-酮戊二酸的产生影响了细胞功能,包括增加对Aβ肽的清除以及表观遗传和基因转录的改变。这一途径在小鼠和人类细胞之间是保守的。关键的是,我们可以使用NLRP3特异性抑制剂在药理学上模拟这种效果,但只能用于慢性NLRP3抑制。总之,这些数据证明了NLRP3的另一个作用,它可以调节线粒体和代谢功能,对阿尔茨海默病的进展具有重要的下游影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


