An oral tricyclic STING agonist suppresses tumor growth through remodeling of the immune microenvironment
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Stimulator of interferon genes (STING) agonists could overcome immunosuppressive microenvironment to improve cancer immunotherapy. However, it is challenging to develop oral STING agonists to achieve systemic immunity. In this study, we discovered ZSA-51 as a potent oral STING agonist with distinct benzo[4,5]thieno[2,3-c]pyrrole-1,3-dione scaffold through tricyclic scaffold screening. ZSA-51, as a prodrug, exhibited nanomolar in vitro STING activation activity and potent in vivo antitumor efficacy in both colon and pancreatic cancer models. The specificity of ZSA-51 in activating STING was confirmed using STING knockout cells and a structurally similar but negative control compound. Moreover, ZSA-51 demonstrated superior oral pharmacokinetic (PK) properties with low toxicity. Importantly, ZSA-51 remodeled immune microenvironment both in tumor and lymph node. Our data suggest that ZSA-51 is a potent oral STING agonist with robust anticancer efficacy, superior PK properties, and low toxicity, holding potential for future development for cancer immunotherapy.

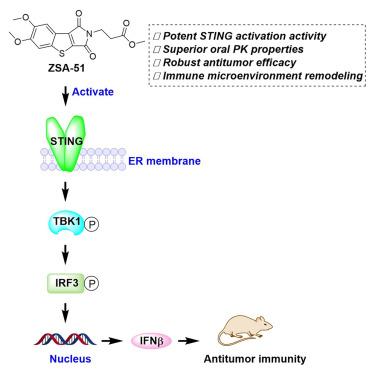
口服三环STING激动剂通过重塑免疫微环境抑制肿瘤生长
干扰素基因刺激剂(STING)激动剂可以克服免疫抑制微环境,改善肿瘤免疫治疗。然而,开发口服STING激动剂以实现全身免疫是具有挑战性的。在本研究中,我们通过三环支架筛选,发现ZSA-51是一种有效的口服STING激动剂,具有独特的苯并[4,5]噻吩[2,3-c]吡咯-1,3-二酮支架。ZSA-51作为前药,在结肠癌和胰腺癌模型中均表现出纳米级体外STING激活活性和强大的体内抗肿瘤功效。ZSA-51激活STING的特异性通过敲除STING细胞和一种结构相似但阴性的对照化合物得到证实。此外,ZSA-51表现出良好的口服药代动力学(PK)特性和低毒性。重要的是,ZSA-51在肿瘤和淋巴结中都重塑了免疫微环境。我们的数据表明ZSA-51是一种有效的口服STING激动剂,具有强大的抗癌功效,优越的PK特性和低毒性,具有未来开发癌症免疫治疗的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


