Spatial transcriptomics identifies molecular niche dysregulation associated with distal lung remodeling in pulmonary fibrosis
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
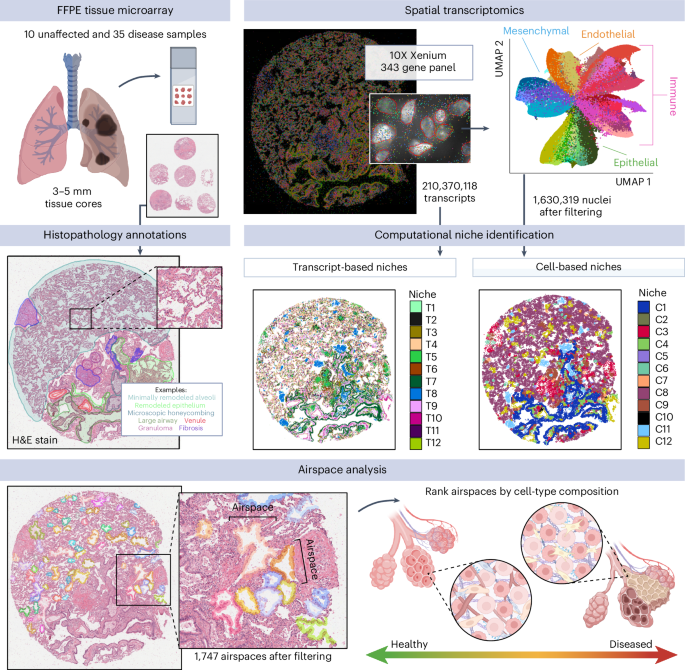
Large-scale changes in the structure and cellular makeup of the distal lung are a hallmark of pulmonary fibrosis (PF), but the spatial contexts that contribute to disease pathogenesis have remained uncertain. Using image-based spatial transcriptomics, we analyzed the gene expression of 1.6 million cells from 35 unique lungs. Through complementary cell-based and innovative cell-agnostic analyses, we characterized the localization of PF-emergent cell types, established the cellular and molecular basis of classical PF histopathologic features and identified a diversity of distinct molecularly defined spatial niches in control and PF lungs. Using machine learning and trajectory analysis to segment and rank airspaces on a gradient of remodeling severity, we identified compositional and molecular changes associated with progressive distal lung pathology, beginning with alveolar epithelial dysregulation and culminating with changes in macrophage polarization. Together, these results provide a unique, spatially resolved view of PF and establish methods that could be applied to other spatial transcriptomic studies. Xenium spatial transcriptomic profiling of pulmonary fibrosis characterizes cell composition dynamics and histopathological features associated with the disease.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


