Structural basis for substrate binding, catalysis and inhibition of cancer target mitochondrial creatine kinase by a covalent inhibitor
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
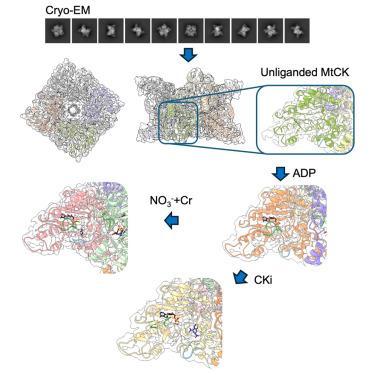
Mitochondrial creatine kinases (MtCKs) are key players in maintaining energy homeostasis in cells that work with cytosolic creatine kinases for energy transport from mitochondria to cytoplasm. The inhibition of breast cancer growth by cyclocreatine targeting CKs indicates dependence of cancer cells on the “energy shuttle” for cell growth and survival. Hence, understanding key mechanistic features of creatine kinases and their inhibition plays an important role in the development of cancer therapeutics. Herein, we present mutational and structural investigations on understudied ubiquitous MtCK that showed closure of the loop comprising His61 is specific to and relies on creatine binding and mechanism of phosphoryl transfer depends on electrostatics of active site. We demonstrate that previously identified pan-CK covalent inhibitor CKi inhibit breast cancer cell proliferation; however, our biochemical and structural data indicated that inhibition by CKi is highly dependent on covalent link formation and conformational changes upon creatine binding are not observed.
共价抑制剂结合底物、催化和抑制癌靶线粒体肌酸激酶的结构基础
线粒体肌酸激酶(mtck)是维持细胞能量稳态的关键角色,它与细胞质肌酸激酶一起将能量从线粒体转运到细胞质。环肌酸对乳腺癌生长的抑制作用表明癌细胞依赖于“能量穿梭”细胞生长和存活。因此,了解肌酸激酶的关键机制特征及其抑制作用在癌症治疗的发展中起着重要作用。在本文中,我们对未被充分研究的普遍存在的MtCK进行了突变和结构研究,表明包含His61的环的关闭特定于并依赖于肌酸结合,磷酸化转移的机制取决于活性位点的静电。我们证明了先前鉴定的泛ck共价抑制剂CKi抑制乳腺癌细胞增殖;然而,我们的生化和结构数据表明,CKi的抑制作用高度依赖于共价键的形成,而肌酸结合时的构象变化未被观察到。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


