Constraints on the location of the liquid–liquid critical point in water
IF 17.6
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
Abstract
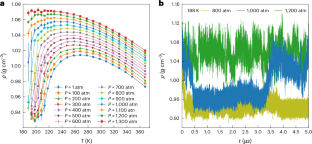
The fascinating hypothesis that supercooled water may segregate into two distinct liquid phases, each with unique structures and densities, was first posited in 1992. This idea, initially based on numerical analyses with the ST2 water-like empirical potential, challenged the conventional understanding of water’s phase behaviour at the time and has since intrigued the scientific community. Over the past three decades, advancements in computational modelling—particularly through the advent of data-driven many-body potentials rigorously derived from first principles and augmented by the efficiency of neural networks—have greatly enhanced the accuracy of molecular simulations, enabling the exploration of the phase behaviour of water with unprecedented realism. Our study leverages these computational advances to probe the elusive liquid–liquid transition in supercooled water. Microsecond-long simulations with chemical accuracy, conducted over several years, provide compelling evidence that water indeed exists in two discernibly distinct liquid states at low temperature and high pressure. By pinpointing a realistic estimate for the location of the liquid–liquid critical point at ~198 K and ~1,250 atm, our study not only advances the current understanding of water’s anomalous behaviour but also establishes a basis for experimental validation. Importantly, our simulations indicate that the liquid–liquid critical point falls within temperature and pressure ranges that could potentially be experimentally probed in water nanodroplets, opening up the possibility for direct measurements. A liquid–liquid transition in supercooled water has long been predicted. State-of-the-art simulations now precisely confine the temperature and pressure ranges for this transition, which are found to be within experimental reach.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


