Vanadium induces Ni-Co MOF formation from a NiCo LDH to catalytically enhance the MgH2 hydrogen storage performance
IF 13.8
1区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
Magnesium-based hydrogen storage materials, such as MgH2, have attracted considerable attention because of its superior hydrogen storage capacities, inexpensive, and excellent reversibility. However, their high thermodynamic stabilities and slow kinetics lead to relatively high desorption temperatures, which severely limit the wide application of MgH2. In this study, the inclusion of vanadium induced the formation Ni-Co metal–organic frameworks (MOF) from a NiCo layered double hydroxide (LDH), thereby increasing the number of defects and vacancies, and improving the hydrogen storage properties of MgH2. The synthesized NiCo-MOF/V-O-doped MgH2 system demonstrates excellent hydrogen storage capacity. More specifically, 5 wt.% of H2 was released over 20 min at a relatively low dehydrogenation temperature of 250 °C, and almost complete dehydrogenation was achieved at 300 °C for 5 min. In addition, at 125 °C, the hydrogen storage material absorbed 5.5 wt.% H2 in 10 min. Furthermore, the activation energy of dehydrogenation was determined to be 69.588 ± 6.302 kJ ·mol−1 which is significantly lower than that of the ball-milled MgH2 (i.e., 118.649 ± 2.825 kJ ·mol−1). It was therefore inferred that during dehydrogenation process, a Mg2Ni/Mg2NiH4 hydrogen pump is formed by Ni, while the V-H and Co-H bonds formed by Co and V during the reaction act synergistically to catalyze the absorption and desorption of hydrogen, thereby increasing the hydrogen storage capacity of MgH2. These experiments provide new perspectives on the commercial application of MgH2.
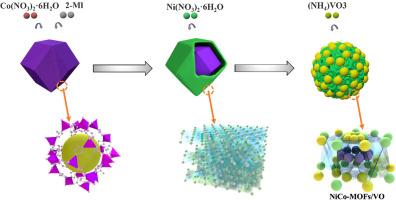
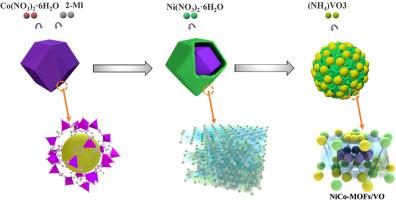
钒在NiCo LDH中诱导Ni-Co MOF形成,催化提高MgH2储氢性能
镁基储氢材料,如MgH2,因其优越的储氢能力、廉价和优异的可逆性而受到广泛关注。然而,由于其较高的热力学稳定性和较慢的动力学特性,导致其解吸温度较高,严重限制了MgH2的广泛应用。在本研究中,钒的加入诱导NiCo层状双氢氧化物(LDH)形成Ni-Co金属有机框架(MOF),从而增加了缺陷和空位的数量,提高了MgH2的储氢性能。合成的NiCo-MOF/ v - o掺杂MgH2体系具有良好的储氢性能。更具体地说,5 wt. %的H2被释放在20分钟250°C的脱氢温度相对较低,而且几乎完全实现脱氢在300°C 5分钟。另外,在125°C,储氢材料吸收5.5 wt. % H2在10分钟。此外,脱氢的活化能是确定为69.588±6.302 kJ·摩尔−1显著低于球磨MgH2(例如,118.649±2.825 kJ·摩尔−1)。由此推断,在脱氢过程中,Ni形成Mg2Ni/Mg2NiH4氢泵,而Co和V在反应过程中形成的V- h键和Co- h键协同作用,催化氢的吸附和解吸,从而增加了MgH2的储氢能力。这些实验为MgH2的商业应用提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Magnesium and Alloys
Engineering-Mechanics of Materials
CiteScore
20.20
自引率
14.80%
发文量
52
审稿时长
59 days
期刊介绍:
The Journal of Magnesium and Alloys serves as a global platform for both theoretical and experimental studies in magnesium science and engineering. It welcomes submissions investigating various scientific and engineering factors impacting the metallurgy, processing, microstructure, properties, and applications of magnesium and alloys. The journal covers all aspects of magnesium and alloy research, including raw materials, alloy casting, extrusion and deformation, corrosion and surface treatment, joining and machining, simulation and modeling, microstructure evolution and mechanical properties, new alloy development, magnesium-based composites, bio-materials and energy materials, applications, and recycling.
文献相关原料
公司名称
产品信息
阿拉丁
Ammonium metavanadate
阿拉丁
Ethanol
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


