Nonaqueous G-Quadruplex Ionogels
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
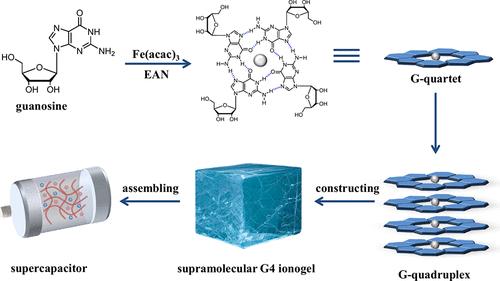
Supramolecular ionogels, as an emerging class of materials, utilize the intriguing properties of ionic liquids (ILs) and offer a promising method for constructing functional materials in anhydrous solvents. G-Quadruplex, assembled from guanine-rich nucleic acid building blocks, is an important structure closely associated with life and leads to the gelation for constructing versatile materials, such as the electrolytes of supercapacitors. Herein, we design and fabricate G-quadruplex ionogels using an unreported cation. Among the metal acetylacetonates under investigation, iron(III) acetylacetonate is distinctive in its capacity to stabilize G-quadruplexes through a reaction with the solvent ethylammonium nitrate (EAN), resulting in the formation of Fe(acac)2+. The ionogel displays high ionic conductivity (6.43 mS·cm–1), a wide electrochemical stability window (2.86 V), and self-healing properties. The supercapacitor based on the G-quadruplex ionogel electrolyte delivers a gravimetric capacitance of 91.8 F·g–1, a high energy density of 32.6 W h/kg, and a power density of 319.8 W/kg at a current density of 0.2A·g–1. Therefore, the present findings have important implications for deepening the understanding of G-quadruplex structures and metal acetylacetonate complexes and provide an approach to fabricating ionogel electrolytes for high-performance supercapacitors.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


