Approach to Enantiomer Recognition during Adsorption by Different Adsorption Rates on Zeotype Borophosphate LiCu2[BP2O8(OH)2] with Spontaneously Emerged Chirality
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
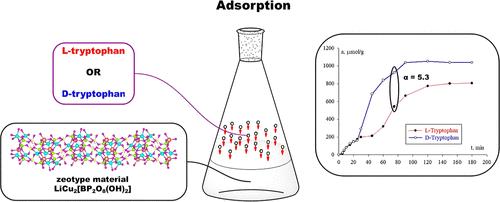
Zeotype borophosphates are porous adsorbents with a 3D open framework. The unique feature of borophosphates is noncentrosymmetric or even chiral structures. The chirality emerged as an asymmetrical arrangement of atoms in crystals and can be referred to the supramolecular, not molecular, chirality hierarchy level. In this work, the zeotype copper borophosphate LiCu2[BP2O8(OH)2] was studied as a promising adsorbent for enantiomer separation. LiCu2[BP2O8(OH)2] has a rare feature: without any external source of chirality, crystals with only [CD(−)284] and [CD(−)760] Cotton effects are synthesized. Tryptophan enantiomer adsorption on LiCu2[BP2O8(OH)2] was studied. At almost all tryptophan concentrations, enantioselectivity was observed. At nonequilibrium conditions, enantioselectivity coefficients were higher than at adsorption equilibrium. In some cases, chiral recognition was not observed at equilibrium but occurred before equilibrium. In a nonequilibrium area of amount of adsorbed substance vs adsorption time curve, a phenomenon of very high enantioselectivity was discovered. The enantioselectivity coefficient values were 3–5, and even 11.8. Such extremely high enantioselectivity values were caused by the nontrivial kinetic curve for l-tryptophan. A far from the equilibrium, a plateau was observed on the curve. At the end of the plateau, the maximal enantioselectivity was observed. This mechanism of enantioselectivity enhancement can be useful for chromatographic separations, chemical sensors, and catalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


