Heterogeneous Nickel Molybdenum Oxide Nanorods Conjugated Graphitic Carbon Nitride: A Bifunctional Electrocatalyst for Overall Water Splitting
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
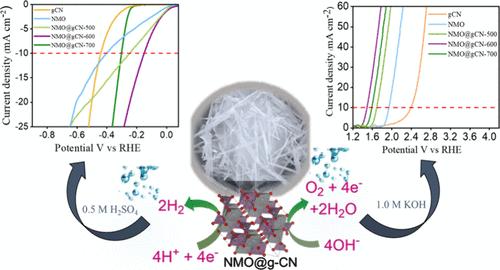
The pursuit of sustainable energy solutions has driven extensive research into efficient and cost-effective water-splitting techniques. This study introduces a straightforward method employing nickel molybdenum oxide NiMoO4 (NMO) nanorods integrated with graphitic carbon nitride (g-CN) sheets as promising catalysts for water splitting. The integrated coupling between NMO nanorods and g-CN leverages the distinctive properties of both materials to boost robustness as well as effectiveness in catalysis for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). We systematically optimized the nanostructure by adjusting the reduction annealing temperature during calcination to improve HER and OER activities. The NMO@g-CN-600 nanostructured catalyst demonstrates exceptional electrochemical HER activity in acidic media, with an overpotential of 148 mV at 10 mA cm–2 current density, which is approximately 2.72 times lower than that of bare NMO and 2.97 times lower than pristine g-CN catalysts. Under alkaline conditions, the NMO@g-CN-600 nanostructured catalyst exhibited superior OER activity with an overpotential of 252 mV to reach a current density of 10 mA cm–2, outperforming bare NMO and pristine g-CN catalysts. Additionally, the nanostructured catalyst demonstrated excellent long-term electrochemical stability with chronoamperometric testing over 50 h in both basic and acidic environments, showing low Tafel slopes for the OER and HER of 97 and 98 mV dec–1, respectively. Various analytical methods confirmed the successful synthesis and structural stability of prepared catalysts. The outstanding electrocatalytic properties of the NMO@g-CN-600 nanostructured catalyst position it as a feasible choice to platinum-group-based catalysts for overall water electrolysis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


