Functional Nanosheet Immunoswitches Reprogramming Innate Macrophages for Immunotherapy of Colorectal Cancer and Sepsis
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
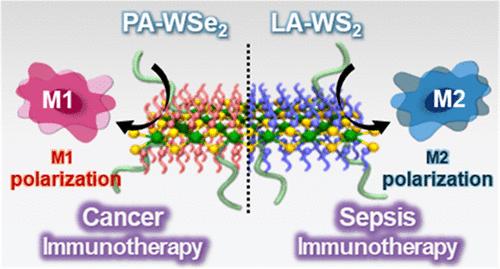
Macrophages are involved in the immunopathogenesis of cancer and inflammatory diseases and are a primary target for immunotherapy to reprogram the M1 and M2 phenotypes in tumor and inflammatory microenvironments. Herein, functional nanosheet immunoswitches that can bidirectionally polarize macrophages in tumor and inflammatory microenvironments are designed for effective immunotherapy of colorectal cancer and sepsis. WSe2 nanosheets are functionalized with palmitic acid to obtain an M1 immunoswitch (PA-WSe2) that promotes the polarization of macrophages toward the M1 phenotype in the tumor microenvironment by activating the STAT1 signaling pathway. WS2 nanosheets bearing linoleic acid are synthesized as an M2 immunoswitch (LA-WS2) that effectively polarizes macrophages to the M2 phenotype in the septic microenvironment by activating the STAT3 signaling pathway. The PA-WSe2 M1 immunoswitch upregulates the secretion of pro-inflammatory cytokines and reactive oxygen and nitrogen species (ROS and RNS) via M1 polarization, leading to the effective immunotherapy for colorectal cancer in vivo. In contrast, the LA-WS2 M2 immunoswitch induces the elevated production of anti-inflammatory cytokines and scavenging of ROS and RNS through M2 polarization, resulting in superior immunotherapy for severe sepsis in mice. These nanosheet immunoswitches can provide a route to immunotherapy for various cancers and inflammatory diseases.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


