Controlled Cooperativity of Proton Tunneling in a Water Trimer
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Proton tunneling through a hydrogen bond is a significant quantum phenomenon in proton-mediated processes. Hydrogen bonds strengthen each other through cooperative interactions, enhancing proton tunneling. Controlling cooperativity of the hydrogen-bond network is required to understand the role of cooperativity in proton tunneling; however, engineering hydrogen bonds is difficult due to the stable structure of hydrogen-bonded cluster. Here, we demonstrate that collective proton tunneling can be controlled inside a cyclic water trimer simply by assigning an asymmetry in the adsorption structure. Asymmetric configuration of water trimers in registry with the NaCl(001) surface perturbs the strength of hydrogen bonds, destroying cooperativity. We reveal two pathways that facilitate proton tunneling in the interfacial trimer: vibration-excited and rotation-mediated processes. The vibrationally excited states lead to lowering the tunneling barrier, and the intermolecular rotation increases the cooperativity by modifying the adsorption configuration. Our results highlight the atomic-scale control of hydrogen bonds, which is crucial in proton-involved reactions.
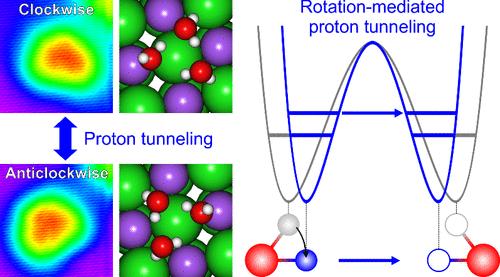
水三聚体中质子隧穿的受控协同性
质子通过氢键进行隧穿是质子介导过程中的一种重要量子现象。氢键通过协同作用相互加强,从而增强质子隧穿。要了解合作性在质子隧穿中的作用,就必须控制氢键网络的合作性;然而,由于氢键簇的结构稳定,氢键工程学很难实现。在这里,我们证明了只需在吸附结构中赋予不对称,就能控制环状水三聚体内部的集体质子隧道。水三聚体与 NaCl(001) 表面的不对称配置扰乱了氢键的强度,破坏了合作性。我们揭示了促进质子在界面三聚体中隧穿的两种途径:振动激发过程和旋转介导过程。振动激发态降低了隧穿屏障,而分子间旋转则通过改变吸附构型提高了合作性。我们的研究结果凸显了氢键的原子尺度控制,这在质子参与的反应中至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


