One-step high-efficiency recovery of high-purity MoO3 from spent hydrodesulfurization catalyst by water-vapor enhanced sublimation process
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
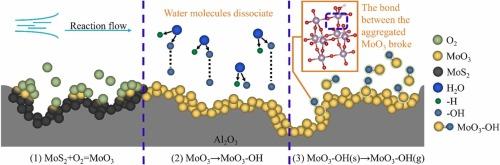
Spent hydrodesulfurization (HDS) catalysts were considered as a vital secondary resource for precious metals like Mo. The current pyrometallurgy and hydrometallurgy usually exhibits the characteristic of remarkable high energy consumption and high secondary pollutions. This study proposes an innovative technique to recycling MoO3 from spent HDS catalyst by sublimation process at high temperature, which has notable advantages of zero wastewater-generation and zero chemical reagent consumption. Notably, MoO3 recovery efficiency was improved remarkably with the introduction of water-vapor. About 99.33% of MoO3 was recovered by heating spent HDS catalyst at 1100℃ for 2.5 h in water-vapor atmosphere with partial pressure of 101.33 kPa. The yielded MoO3 was tested with the purity of 99.94% and exhibited the appearance of thin strips. Furthermore, the sublimation kinetic of MoO3 in air was adhered to a desorption model, while agreed with a non-desorption model in water-vapor atmosphere. Density Functional Theory (DFT) calculations revealed that -OH obtained by the dissociation of H2O molecules preferably combined with MoO3 and formed the volatile MoO3-OH, which was responsible for enhancing MoO3 sublimation efficiency significantly in water-vapor atmosphere. Economic analysis suggested that the direct cost of this method was 345 $/t, accounting for around 50% compared to current roasting-leaching-purification methods. Overall, MoO3 sublimation enhanced by water-vapor atmosphere can be considered as a high-efficient and environmental-friendly approach for Mo recovery from spent catalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


