Targeted and Synergistic Codelivery of Chemotherapeutic and Nucleic Acid Drugs by Liposome-Coated MPDA Nanoparticles for Advanced Prostate Cancer Treatment
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
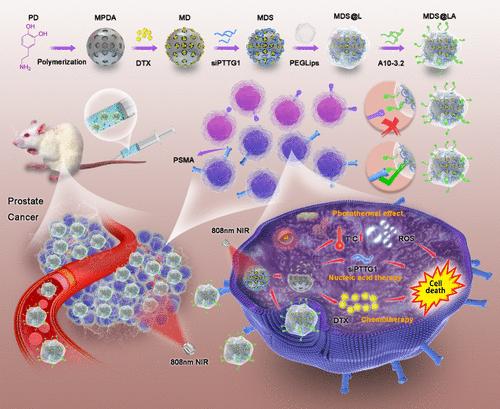
Docetaxel (DTX)-based chemotherapy is the primary therapeutic approach for advanced prostate cancer (PCa) when endocrine therapy proves ineffective. Traditional chemotherapy exhibits poor specificity and induces severe side effects, such as immunosuppression, neurotoxicity, and hypersensitivity. In this study, we aimed to develop a new targeted nanodrug delivery system to accurately identify PCa cells and deliver drugs. We prepared mesoporous polydopamine (MPDA) nanoparticles using a one-pot method. After loading DTX onto MPDA, siRNA was attached to the surface, which was coated with polyethylene glycol lipids film (PEG-Lips); together, this formed MDS@L. The aptamer A10-3.2 was coupled to the surface of PEG-Lips to obtain MDS@LA, which was characterized using different techniques, including transmission electron microscopy and Fourier transform infrared spectroscopy. MDS@LA exhibited excellent stability, acid-responsive release, and photothermal properties, enhancing its antitumor effects. Both in vitro and in vivo experiments revealed that MDS@LA precisely targeted PCa cells and effectively delivered DTX and siRNA, leading to significant inhibition of PCa cell growth and proliferation. This versatile nanoplatform offers a promising, precise, and efficient therapeutic approach for advanced PCa, addressing the limitations of conventional chemotherapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


