Dissecting the Essential Role of a Molecular Promoter C60 on a Ru Catalyst for Ammonia Synthesis
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
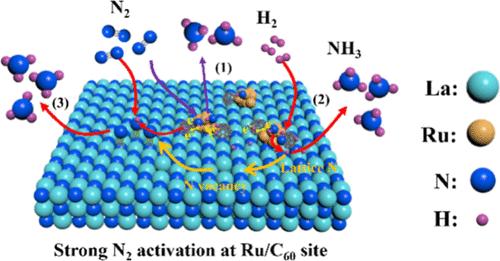
The major bottlenecks for ammonia (NH3) synthesis under mild conditions are the activation of the extremely inert N≡N bond (941 kJ mol–1) and/or the desorption of NH3 from the catalyst surface. Electron donation from the appropriate promoters is essential to enhance N2 activation over Fe or Ru catalysts. Nevertheless, despite typical element promoters enhancing the N2 activation efficiency, they induce strong NH3 binding on the catalyst surface, leading to the need for high temperatures and pressures for the reaction to complete. Herein, we propose the use of a molecular promoter (C60) to tackle the difficulties. The positioning role of C60 at a 1 nm scale on small Ru nanoclusters drives the exposure of more terrace sites (geometric effect) and induces d–π interactions at the Ru–C60 junctions. The latter electronically modifies the Ru sites (electronic effect), thereby synergistically contributing to N2 and H2 activation as well as to the release of NH3 on the Ru sites. The significant electron buffer attribute of C60 and the strong electronic interaction between Ru and C60 facilitate a shift of d-band center toward the Fermi level and a decrease of work function, simultaneously satisfying the electronic requirements for N2 activation enhancement and NH3 binding weakening. Consequently, the C60-promoted Ru/LaN catalyst exhibits a high NH3 synthesis rate. It is envisioned that our findings have significant implications for the rational search of molecular promoters for high-efficiency NH3 synthesis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


