Experimental and Theoretical Thermokinetic Analysis of the Na3VO4–CO2 Reaction System under Different Physicochemical Conditions
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
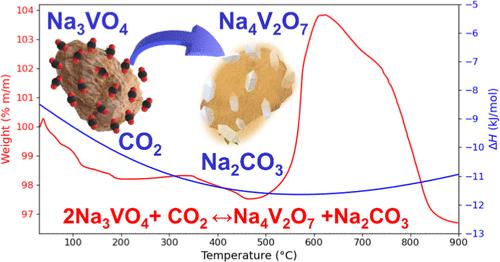
Sodium vanadate (Na3VO4) was synthesized, structural and microstructurally characterized as well as tested as possible carbon dioxide (CO2) captor through thermodynamic calculations in addition to thermogravimetric dynamic and isothermal analyses. Ceramic structural characterization evidenced the formation of the Na3VO4 crystal phase, while microstructural features evidenced dense agglomerates with a poor specific surface area, although the synthesis temperature (600 °C) was not as high as that for other sodium ceramics. Na3VO4 was investigated for the CO2 capture process through dynamic and isothermal experiments in the presence or absence of oxygen. All of these experiments showed that Na3VO4 possesses interesting CO2 capture properties in a specific temperature range (520 and 600 °C). Isothermal product characterization allowed us to elucidate the reaction pathway, implying the Na4V2O7 formation, as possible reactive intermediate. In fact, reaction path evolution was supported with theoretical thermodynamic data. Moreover, the kinetic analysis, performed using the Avrami-Erofeev mathematical model and subsequent thermodynamic data obtention, probed the positive influence of the temperature in both the CO2 superficial and bulk chemical sorption processes, while oxygen addition did not enhance the superficial process. Additionally, as would be expected, kinetics were enhanced as a function of the CO2 concentration.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


