Radiation-induced impacts on mitochondrial DNA and the transgenerational genomic instability
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Background
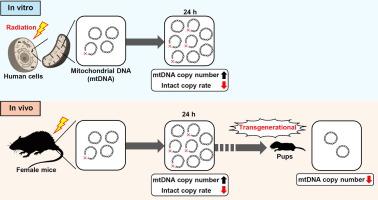
Mitochondrial genomes are dynamically evolving and are shaped by somatic mutation and selection throughout the female germline. In this study, we investigated the radiation-induced impacts on mitochondrial DNA in vitro and in vivo, as well as the transgenerational inheritance.Methods
Human cervical cancer HeLa cells and telomerase-immortalized normal fibroblast BJ1-hTERT cells were exposed to X-rays at 0.5–8 Gy. Also, we exposed 8-week-old female C57BL/6N mice to a single whole-body dose of 2 Gy of X-rays and mated them with healthy males 1 day after irradiation. We extracted DNA from the irradiated cells, female mice, and the 2-week-old pups, then examined the mitochondrial DNA copy numbers (mtDNAcns) and radiation-induced damage by real-time quantitative PCR.Results
The mtDNAcn levels in cells increased and the intact copy ratios decreased 24 h after irradiation, resulting in the delayed shift of heteroplasmy. Also, the peripheral blood-derived mtDNAcn levels in irradiated females increased 1 day after irradiation and the intact copy rates decreased, supporting the results in vitro. Furthermore, whole blood-derived mtDNAcn levels decreased in 2-week-old pups born from irradiated mothers, indicating the mitochondrial genomic instability.Discussion
We found the delayed induction of mtDNA damage in human cancer and noncancerous cells after irradiation, as well as the same trend in mice. Furthermore, to our knowledge, this is first to demonstrate the hereditary effects of radiation on the regulation of mitochondrial genome. These provide novel insights into the significance of radiation protection and preventive medicine for not only pre-pregnancy females but also the next generation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


