Design and synthesis of novel sulfur-substituted triptolide with the ability to induce autophagy through inhibition of SRSF1 expression
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
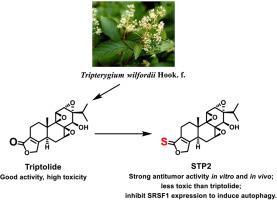
Six sulfur-substituted triptolide (TPL) analogs (STP1-6) were synthesized and evaluated for their biological functions. Among them, STP2 had significant antitumor activity both in vitro and in vivo. Notably, the intraperitoneal injections of 1 g/kg STP2 did not cause mice death and apparent pathological damage, while the mice in the TPL group (2 mg/kg) lost weight and all died within 4 days. The antitumor effect of STP2 could mediated by the inhibition of SRSF1 expression to regulate Bcl-x pre-mRNA splicing, which in turn induces autophagy and promotes cell death. This mechanism was the first time discovered in the field of TPL research. These results indicated that compound STP2 could be a promising lead compound for further studies.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


