Long-term imaging of individual ribosomes reveals ribosome cooperativity in mRNA translation
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The genetic information stored in mRNAs is decoded by ribosomes during mRNA translation. mRNAs are typically translated by multiple ribosomes simultaneously, but it is unclear whether and how the activity of different ribosomes on an mRNA is coordinated. Here, we develop an imaging approach based on stopless-ORF circular RNAs (socRNAs) to monitor translation of individual ribosomes in either monosomes or polysomes with very high resolution. Using experiments and simulations, we find that translating ribosomes frequently undergo transient collisions. However, unlike persistent collisions, such transient collisions escape detection by cellular quality control pathways. Rather, transient ribosome collisions promote productive translation by reducing ribosome pausing on problematic sequences, a process we term ribosome cooperativity. Ribosome cooperativity also reduces recycling of ribosomes by quality control pathways, thus enhancing processive translation. Together, our single-ribosome imaging approach reveals that ribosomes cooperate during translation to ensure fast and efficient translation.
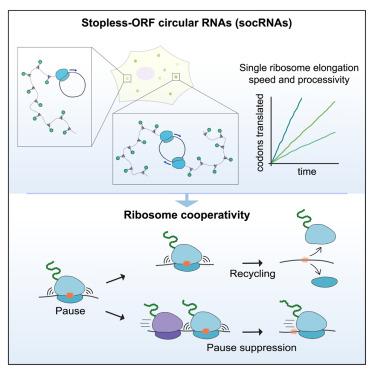
单个核糖体的长期成像揭示了核糖体在mRNA翻译中的协同性
储存在mRNA中的遗传信息在mRNA翻译过程中被核糖体解码。mRNA通常由多个核糖体同时翻译,但目前尚不清楚不同核糖体对mRNA的活性是否以及如何协调。在这里,我们开发了一种基于无间断orf环状rna (socRNAs)的成像方法,以非常高的分辨率监测单体或多体中单个核糖体的翻译。通过实验和模拟,我们发现翻译核糖体经常发生瞬态碰撞。然而,与持续的碰撞不同,这种瞬态碰撞逃脱了细胞质量控制途径的检测。相反,短暂的核糖体碰撞通过减少核糖体在有问题的序列上的停顿来促进有效的翻译,我们称之为核糖体合作的过程。核糖体的协同性还通过质量控制途径减少了核糖体的再循环,从而增强了过程翻译。总之,我们的单核糖体成像方法揭示了核糖体在翻译过程中的合作,以确保快速有效的翻译。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


