Palladium-catalyzed carbonylative synthesis of 13C-labeled flavones
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
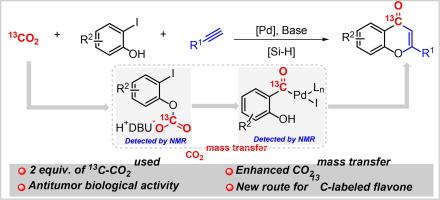
13C-labeled compounds are invaluable in drug discovery as lead compounds and as tracers for studying drug bioavailability and pharmacodynamics. However, these approaches are mainly limited to high-value chemical labeling materials and low atom economy multistep synthesis. Herein, we report a highly efficient Pd-catalyzed reductive carbonylation method for the synthesis of 13C-labeled flavones using near-stoichiometric [13C]CO2. With aromatic alkynes, aliphatic alkynes and substituted 2-iodophenols as the reaction partners, the corresponding 13C-labeled flavones were obtained in good yields with excellent functional group tolerance. Mechanistic studies suggest that the phenolic group accelerates CO2 mass transfer, followed by intramolecular migration and the formation of key acylpalladium intermediates. Notably, para-tBu substituted 13C-flavone results in a more pronounced enhancement of antitumor efficacy compared to ester or OMe substitutions, relative to their 12C analogs.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


