The Crucial Role of Lewis Basicity of N-Heterocyclic Carbenes in the CO2 Hydroboration Reduction: Comprehensive Insights from Density Functional Theory Calculations and Microkinetic Simulations
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
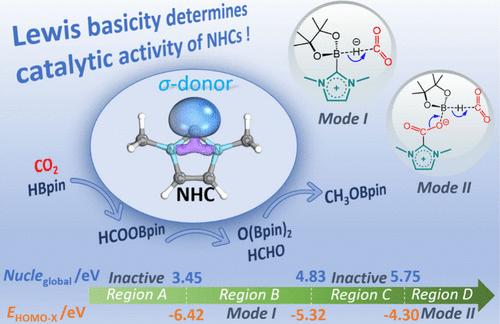
The hydroboration reduction of CO2 by HBpin, catalyzed by N-heterocyclic carbene (NHC), has been investigated using density functional theory (DFT) calculations and microkinetic simulations. NHC acts as an effective activator for both CO2 and HBpin, and thus, NHC and its CO2 adduct, NHC–CO2, function as crucial active catalysts in the hydroboration reduction of CO2. Herein, the preferences of these two active catalysts in the hydroboration of CO2 have been evaluated, and the catalytic reaction mechanism has been unveiled. A linear relationship between carbene-type catalysts and their chemical reactivities is established to screen highly activated carbene species for the CO2 hydroboration reduction. It is found that the chemical reactivities of NHCs, including small molecule activation and CO2 hydroboration reduction, are closely related to their Lewis basicity and global nucleophilicity. In addition, microkinetic simulations have been performed to analyze the influence of initial stoichiometric ratio and temperature on the temporal distribution of various species. The present study suggests a fresh perspective on the application of NHC and its analogues in CO2 reduction.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


