CO2 and H2S Solubility in Acidified Aqueous Mixtures of N-Methyldiethanolamine: Experimental Measurements and Thermodynamic Modeling
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
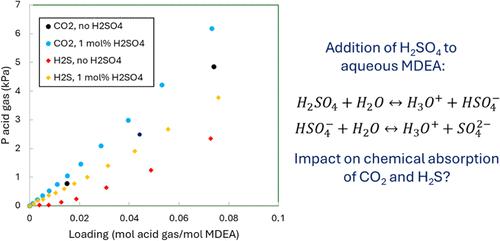
The addition of a strong acid to an aqueous alkanolamine solvent is common in different acid gas removal applications to improve the selectivity toward H2S compared to CO2 and thus reach more stringent H2S specifications. The purpose of solvent acidification is to shift the equilibrium of the reactions. In this work, the vapor–liquid equilibria (VLE) of CO2 and H2S in aqueous solutions of N-methyldiethanolamine (MDEA) with 0.5 and 1.0 mol % sulfuric acid (H2SO4) were measured at temperatures of 323 and 353 K. A reduced solubility for both acid gases was observed for experiments with H2SO4 compared with experiments without acidification. The experimental VLE results were used to develop a thermodynamic model based on e-NRTL. The model accurately predicts the experimental data in this work and from other authors. Additionally, qualitative kinetic experiments were performed, and they have shown limited impact on the absorption rates of both acid gases. The study improves our understanding of the impact of strong acids on thermodynamics and kinetics and on acid gas removal processes at the industrial scale.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


