Binuclear Ni catalyzed ethylene copolymerization with short chain alkenol monomers†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
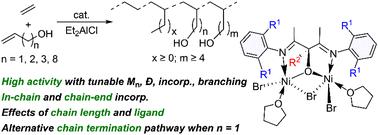
Ethylene coordination copolymerization with vinyl polar monomers, particularly short chain alkenols, offers an attractive method for controlled synthesis of important hydroxy-functionalized polyethylenes under mild conditions. However, reports on short-chain alkenol copolymerization are limited due to issues like chelating coordination and β-O elimination. Here, we report the synthesis and characterization of binuclear Ni complexes for ethylene copolymerization with various alkenol monomers such as allyl-OH, 3-buten-1-ol, 4-penten-1-ol and 9-decen-1-ol. These complexes, upon activation with Et2AlCl, achieved notable activity (as high as 592 kg (mol cat h atm)−1) in ethylene/3-buten-1-ol copolymerization, producing copolymers with 1.7 mol% comonomer incorporation and a high molecular weight (Mn = 64.2 kg mol−1). The activity and comonomer content were influenced by Et2AlCl loading, reaction temperature, and alkenol monomer length, with longer alkenols such as 9-decen-1-ol yielding higher activity, comonomer incorporation and molecular weight. Activities up to 169 kg (mol cat h atm)−1 were also achieved in ethylene/allyl-OH copolymerization with reduced molecular weight (Mn = 17.2 kg mol−1). Microstructural analysis revealed predominant in-chain and chain-end polar monomer incorporation in all cases. Notably, ethylene/allyl-OH copolymers exhibited unique olefinic end groups and microstructures assignable to Friedel–Crafts reactions, which is likely due to an alternative chain termination pathway associated with the short chain length between the O atom and the active Ni center. For comparison, ethylene/allyl-OAc copolymers showed exclusively olefinic groups, indicating a β-OAc elimination mechanism. This process resulted in lower activity and molecular weight, suggesting catalyst poisoning from rapid chain termination.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


