Carbohydrate-active enzymes from Akkermansia muciniphila break down mucin O-glycans to completion
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
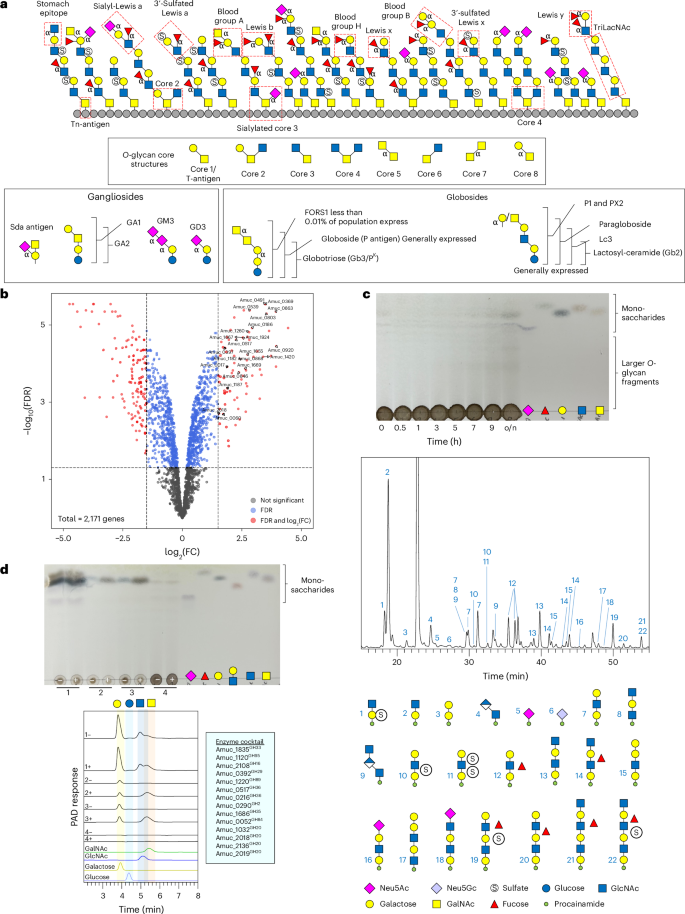
Akkermansia muciniphila is a human microbial symbiont residing in the mucosal layer of the large intestine. Its main carbon source is the highly heterogeneous mucin glycoprotein, and it uses an array of carbohydrate-active enzymes and sulfatases to access this complex energy source. Here we describe the biochemical characterization of 54 glycoside hydrolases, 11 sulfatases and 1 polysaccharide lyase from A. muciniphila to provide a holistic understanding of their carbohydrate-degrading activities. This was achieved using a variety of liquid chromatography techniques, mass spectrometry, enzyme kinetics and thin-layer chromatography. These results are supported with A. muciniphila growth and whole-cell assays. We find that these enzymes can act synergistically to degrade the O-glycans on the mucin polypeptide to completion, down to the core N-acetylgalactosaime. In addition, these enzymes can break down human breast milk oligosaccharide, ganglioside and globoside glycan structures, showing their capacity to target a variety of host glycans. These data provide a resource to understand the full degradative capability of the gut microbiome member A. muciniphila. Biochemical characterization of 66 carbohydrate-active enzymes from the gut microorganism Akkermansia muciniphila reveals that these enzymes can break down a range of host glycans, including mucin, which they degrade to completion.

来自嗜粘菌的碳水化合物活性酶将粘蛋白o -聚糖分解完成
嗜粘阿克曼氏菌是一种居住在大肠粘膜层的人类微生物共生体。它的主要碳源是高度异质性的粘蛋白糖蛋白,它使用一系列碳水化合物活性酶和硫酸酯酶来获取这种复杂的能量来源。本文描述了嗜粘杆菌中54种糖苷水解酶、11种硫酸酯酶和1种多糖裂解酶的生化特性,以全面了解它们的碳水化合物降解活性。这是通过多种液相色谱技术,质谱,酶动力学和薄层色谱实现的。这些结果得到了嗜粘杆菌生长和全细胞实验的支持。我们发现这些酶可以协同作用,降解粘蛋白多肽上的o -聚糖直至完成,直至核心的n -乙酰半乳糖酶。此外,这些酶还能分解人母乳中的低聚糖、神经节苷和glob糖苷聚糖结构,显示出它们针对多种宿主聚糖的能力。这些数据为了解肠道微生物组成员a. muciniphila的完全降解能力提供了资源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


