SNORD113–114 cluster maintains haematopoietic stem cell self-renewal via orchestrating the translation machinery
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
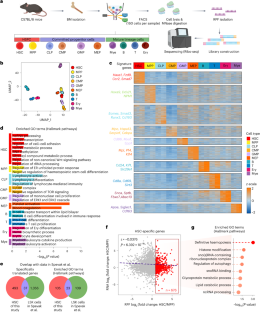
Haematopoietic stem cells (HSCs) self-renew and differentiate to replenish the pool of blood cells, which require a low but finely tuned protein synthesis rate. Nonetheless, the translatome landscape in HSCs and how the translation machinery orchestrates HSC self-renewal remain largely elusive. Here we perform ultra-low-input Ribo-seq in HSCs, progenitor and lineage cells, and reveal HSC-specific translated genes involved in rRNA processing. We systematically profile small nucleolar RNAs (snoRNAs) and uncover an indispensable role of the SNORD113–114 cluster in regulating HSC self-renewal. Maternal knockout (Mat-KO) of this cluster substantially impairs HSC self-renewal, whereas loss of the paternal allele shows no obvious phenotype. Mechanistically, Mat-KO results in dysregulation of translation machinery (rRNA 2′-O-Me modifications, pre-rRNA processing, 60S ribosome assembly and translation) and induces nucleolar stress in HSCs, which exempts p53 from Mdm2-mediated proteasomal degradation and leads to apoptosis. Collectively, our study provides a promising facet to our understanding of snoRNA-mediated regulation in HSC homeostasis. Wang et al. profile the translatome of haematopoietic stem cells (HSCs) and downstream progenitors and lineages. They identify the SNORD113–114 cluster as a modulator of translation and self-renewal in HSCs.

求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


