Lactate homeostasis is maintained through regulation of glycolysis and lipolysis
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Lactate is among the highest flux circulating metabolites. It is made by glycolysis and cleared by both tricarboxylic acid (TCA) cycle oxidation and gluconeogenesis. Severe lactate elevations are life-threatening, and modest elevations predict future diabetes. How lactate homeostasis is maintained, however, remains poorly understood. Here, we identify, in mice, homeostatic circuits regulating lactate production and consumption. Insulin induces lactate production by upregulating glycolysis. We find that hyperlactatemia inhibits insulin-induced glycolysis, thereby suppressing excess lactate production. Unexpectedly, insulin also promotes lactate TCA cycle oxidation. The mechanism involves lowering circulating fatty acids, which compete with lactate for mitochondrial oxidation. Similarly, lactate can promote its own consumption by lowering circulating fatty acids via the adipocyte-expressed G-protein-coupled receptor hydroxycarboxylic acid receptor 1 (HCAR1). Quantitative modeling suggests that these mechanisms suffice to produce lactate homeostasis, with robustness to noise and perturbation of individual regulatory mechanisms. Thus, through regulation of glycolysis and lipolysis, lactate homeostasis is maintained.
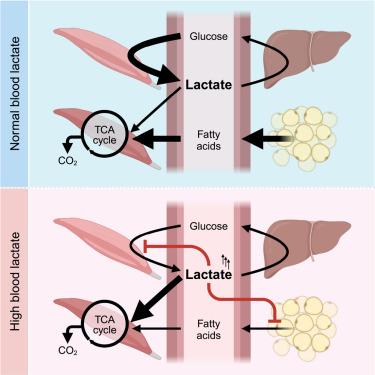
乳酸体内平衡是通过调节糖酵解和脂解来维持的
乳酸是通量最高的循环代谢物之一。它由糖酵解产生,并由三羧酸(TCA)循环氧化和糖异生清除。严重的乳酸水平升高会危及生命,适度的乳酸水平升高预示着未来的糖尿病。然而,乳酸体内平衡是如何维持的,人们仍然知之甚少。在这里,我们在小鼠中发现了调节乳酸产生和消耗的稳态回路。胰岛素通过上调糖酵解诱导乳酸生成。我们发现高乳酸血症抑制胰岛素诱导的糖酵解,从而抑制过量的乳酸生成。出乎意料的是,胰岛素也促进乳酸TCA循环氧化。其机制包括降低与乳酸竞争线粒体氧化的循环脂肪酸。同样,乳酸可以通过脂肪细胞表达的g蛋白偶联受体羟基羧酸受体1 (HCAR1)降低循环脂肪酸,从而促进自身的消耗。定量模型表明,这些机制足以产生乳酸稳态,对噪声和个体调节机制的扰动具有鲁棒性。因此,通过调节糖酵解和脂解,乳酸体内平衡得以维持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


