Loss of SGK1 supports metastatic colonization in hepatocellular carcinoma by promoting resistance to T cell-mediated immunity
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
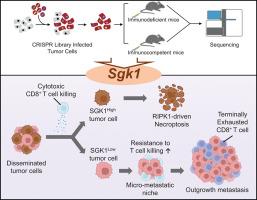
Immune evasion by tumor cells is a principal obstacle to effectively targeting metastasis in hepatocellular carcinoma (HCC). However, the specific molecular mechanisms facilitating immune escape during metastatic seeding are not fully elucidated.Methods
Utilizing in vivo CRISPR library screening in murine HCC metastasis models under conditions of both intact and depleted T cell immunity, we identified genes critical to tumor immune evasion during metastatic colonization, and investigated intrinsic mechanisms by several experimental approaches.Results
Our screens identified Sgk1 as an essential suppressor of metastatic colonization under T cell immunosurveillance. Sgk1-deficient tumor cells displayed significantly enhanced metastatic capacity in the presence of CD8+ T cells, underscoring the role of Sgk1 in regulating immune escape. Clinical analyses corroborated these findings, showing markedly lower SGK1 expression in circulating tumor cells and metastatic lesions relative to matched primary tumors in HCC patients, with low SGK1 expression associating with compromised T cell function and poorer clinical outcomes. Mechanistically, Sgk1 inactivation in tumor cells attenuated CD8+ T cell-mediated, RIPK1-dependent necroptosis—a cell death pathway essential for cytotoxic T cell-mediated restriction of metastasis. Loss of Sgk1 consequently enabled tumor cells to circumvent T cell-induced cytotoxicity, thereby promoting metastatic colonization. Furthermore, the outgrowth of Sgk1-deficient metastatic cells induced a microenvironmental shift toward terminal T cell exhaustion, establishing conditions conducive to sustained immune evasion.Conclusions
These findings establish SGK1 as a crucial regulator of immune-mediated control over metastatic growth in HCC. SGK1 expression in metastatic lesions may serve as a predictive biomarker for response to immune checkpoint inhibitors, presenting new avenues for therapeutic intervention to overcome immune resistance in metastatic HCC.Impact and implications
Despite metastasis is a common occurrence and lethal determinant in cancers, the mechanism underlying tumor immune evasion during metastatic seeding is unclear. Our study reveals that loss of Sgk1 renders metastatic tumor cells survival advantages against antitumor immune response by abrogating CD8+ T cell-induced RIPK1-dependent necroptosis. Growth of Sgk1-silenced metastasis led to infiltration of terminally exhausted CD8+ T cells, which could be reversed by immune checkpoint inhibitors administered at early stage of metastatic seeding. These findings provide valuable insights into potential therapeutic strategies targeting resistance to T cell immunity in cancer metastasis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


