Short IL-18 generated by caspase-3 cleavage mobilizes NK cells to suppress tumor growth
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Interleukin (IL)-18 functions primarily through its 18-kDa mature form produced from caspase-1 cleavage. However, IL-18 can also be processed by other proteases, leading to the generation of different fragments with less recognized functions. Here, we discover that, in cancer cells, caspase-3 cleavage of IL-18 generates a 15-kDa form of IL-18, referred to as short IL-18. Unlike mature IL-18, short IL-18 is not secreted, and does not bind IL-18Rα; instead, it translocates into the nucleus, facilitating STAT1 phosphorylation at Ser727 via CDK8, and enhancing the expression and secretion of ISG15. This signaling cascade in cancer cells mobilizes natural killer cells with increased cytotoxicity to eliminate various syngeneic tumors and colitis-associated colorectal cancer in mice. Moreover, patients with colorectal cancer who have an abundance of short IL-18 in the nucleus have a better prognosis. This work highlights a distinct anti-tumor pathway driven by short IL-18. Here the authors identify a nuclear short form of IL-18 in cancer cells that can enhance NK cell activation to promote tumor elimination.

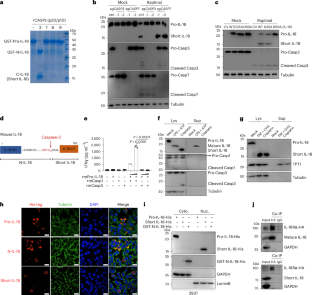
caspase-3裂解产生短IL-18,动员NK细胞抑制肿瘤生长
白细胞介素(IL)-18主要通过caspase-1切割产生的18 kda成熟形式发挥作用。然而,IL-18也可以被其他蛋白酶加工,导致产生不同的片段,功能较少。在这里,我们发现,在癌细胞中,caspase-3切割IL-18产生一个15 kda形式的IL-18,称为短IL-18。与成熟IL-18不同,短IL-18不分泌,也不结合IL-18Rα;相反,它易位到细胞核中,通过CDK8促进STAT1在Ser727位点的磷酸化,并增强ISG15的表达和分泌。癌细胞中的信号级联调动自然杀伤细胞,增加细胞毒性,消除小鼠的各种同基因肿瘤和结肠炎相关结直肠癌。此外,结直肠癌患者在细胞核中具有丰富的短IL-18具有更好的预后。这项工作强调了由短IL-18驱动的独特的抗肿瘤途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


