Progressively differentiated TFH13 cells are stabilized by JunB to mediate allergen germinal center responses
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Allergic diseases are common and affect a large proportion of the population. Interleukin-13 (IL-13)-expressing follicular helper T (TFH13) cells are a newly identified population of TFH cells that have been associated with high-affinity IgE responses. However, the origins, developmental signals, transcriptional programming and precise functions of TFH13 cells are unknown. Here, we examined the developmental signals for TFH13 cells and found a direct and progressive differentiation pathway marked by the production of IL-21. These two pathways differed in kinetics and extrinsic requirements. However, both pathways converged, forming transcriptionally similar TFH13 cells that express the transcription factor JunB as a critical stabilizing factor. Using an intersectional genetics-based TFH13-diphtheria toxin receptor model to perturb these cells, we found that TFH13 cells were essential to drive broad germinal center responses and allergen-specific IgG and IgE. Moreover, we found that IL-21 is a broad positive regulator of allergen germinal center B cells and synergizes with IL-13 produced by TFH13 cells to amplify allergic responses. Thus, TFH13 cells orchestrate multiple features of allergic inflammation. TFH cells that express IL-13 are associated with high-affinity IgE responses, but factors controlling their development, transcriptional programming and exact function have remained unclear. Here, Chandrakar et al. find that the transcription factor JunB is required for TFH13 cell maintenance and that TFH13 cells producing IL-21 drive broad germinal center responses to allergen-specific IgG and IgE.

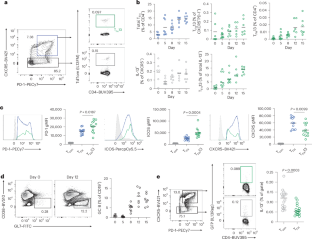
JunB稳定逐渐分化的TFH13细胞,介导过敏原生发中心反应
过敏性疾病很常见,影响很大一部分人口。表达白细胞介素-13 (IL-13)的滤泡辅助性T细胞(TFH13)是一种新发现的与高亲和力IgE反应相关的TFH细胞群。然而,TFH13细胞的起源、发育信号、转录编程和精确功能尚不清楚。在这里,我们研究了TFH13细胞的发育信号,发现了以IL-21的产生为标志的直接和渐进的分化途径。这两种途径在动力学和外在要求上不同。然而,这两种途径融合,形成转录相似的TFH13细胞,表达转录因子JunB作为关键的稳定因子。使用基于交叉遗传学的TFH13-白喉毒素受体模型来干扰这些细胞,我们发现TFH13细胞对于驱动广泛的生发中心反应和过敏原特异性IgG和IgE至关重要。此外,我们发现IL-21是过敏原生发中心B细胞的一个广泛的正调节因子,并与TFH13细胞产生的IL-13协同放大过敏反应。因此,TFH13细胞协调了过敏性炎症的多种特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


