Development and Evaluation of the Dermatomyositis Outcomes for Muscle and Skin as an Outcome Measure in Dermatomyositis Clinical Trials
JID innovations : skin science from molecules to population health
Pub Date : 2024-12-09
DOI:10.1016/j.xjidi.2024.100337
引用次数: 0
Abstract
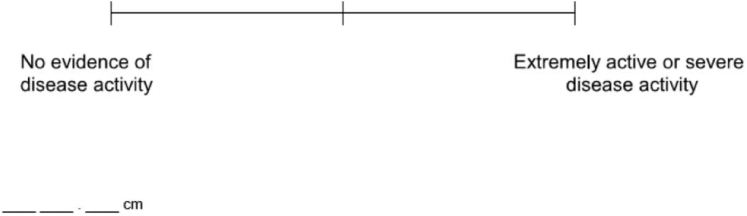
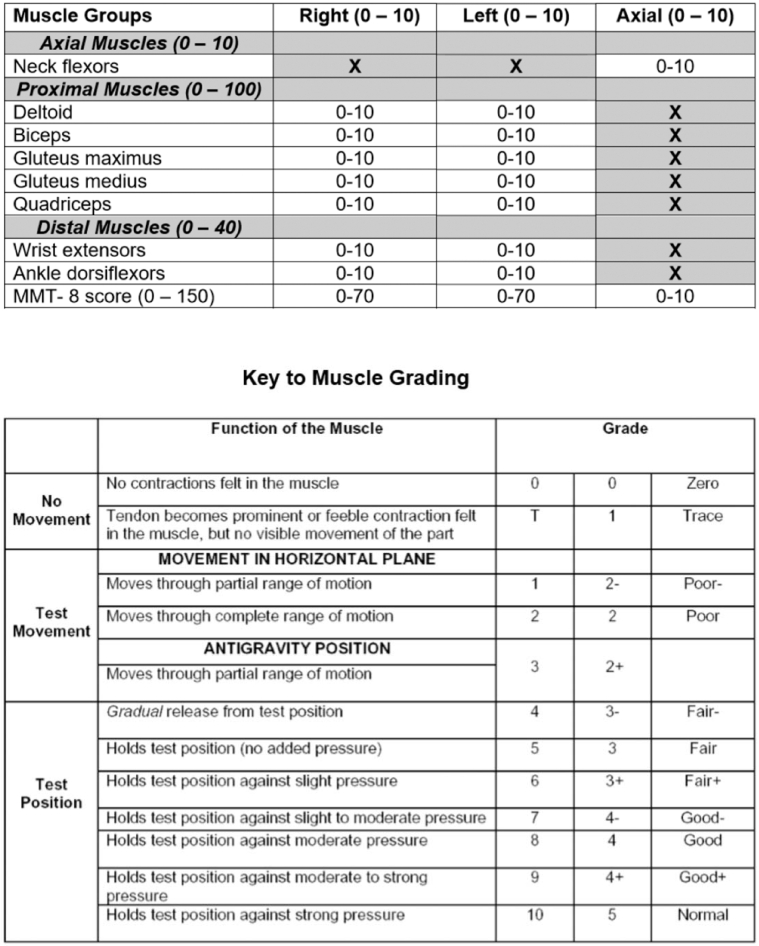
The Total Improvement Score (TIS), which is used as the primary efficacy measure in dermatomyositis (DM) clinical trials, lacks a skin-specific measure. However, skin is a defining feature of DM. In this study, data were analyzed from the phase 3 trial of lenabasum in DM. Cutaneous Dermatomyositis Disease Area and Severity Index-Activity scores and all components of the TIS were collected at baseline and weeks 16, 28, 40, and 52. From these assessments, a composite outcome was developed, named Dermatomyositis Outcomes for Muscle and Skin, which includes certain components of the TIS and the Cutaneous Dermatomyositis Disease Area and Severity Index-Activity scores. The relative sensitivities of the TIS and Dermatomyositis Outcomes for Muscle and Skin to detect improvement in DM skin and muscle disease activity were analyzed. A total of 174 patients with DM were included, 82% were female, and 75% were White. Mean (SD) age was 51.9 (12.20) years. Treatment effect using the TIS ranged between 17.6 and 21.7 points for muscle and skin responders versus nonresponders across time points. The Dermatomyositis Outcomes for Muscle and Skin score displayed a statistically significantly greater treatment effect of 25.9–40.0 points for responders than for nonresponders, depending on the response assessed and the time point. Dermatomyositis Outcomes for Muscle and Skin is a more sensitive composite measure that reflects improvement from baseline in both skin and muscle disease activity, suggesting usefulness for use in future DM clinical trials.

皮肌炎临床试验中肌肉和皮肤皮肌炎结果的发展和评价。
总改善评分(TIS)作为皮肌炎(DM)临床试验的主要疗效指标,缺乏皮肤特异性指标。然而,皮肤是糖尿病的一个决定性特征。在本研究中,分析了lenabasum治疗糖尿病的3期试验数据。皮肤皮肌炎疾病面积和严重程度指数-活动评分以及TIS的所有组成部分在基线和第16、28、40和52周收集。从这些评估中,开发了一个复合结果,称为肌肉和皮肤皮肌炎结果,其中包括TIS和皮肤皮肌炎疾病面积和严重程度指数-活动评分的某些组成部分。分析TIS和皮肌炎预后对检测糖尿病皮肤和肌肉疾病活动改善的相对敏感性。共纳入174例糖尿病患者,82%为女性,75%为白人。平均(SD)年龄为51.9(12.20)岁。使用TIS的治疗效果在肌肉和皮肤反应者与无反应者的时间点之间为17.6至21.7分。根据评估的反应和时间点,肌肉和皮肤皮肌炎结局评分显示,有反应者的治疗效果比无反应者高25.9-40.0分,在统计学上具有显著性。肌肉和皮肤皮肌炎预后是一种更敏感的复合指标,反映了皮肤和肌肉疾病活动从基线开始的改善,表明在未来的糖尿病临床试验中有用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.00
自引率
0.00%
发文量
0
审稿时长
8 weeks

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


