Engineered material-binding peptide empowers biocatalysis in stainless steel flow reactors for phosphate recovery
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
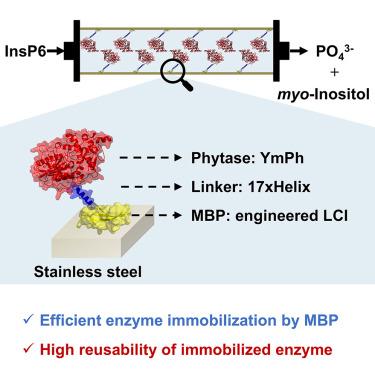
Biocatalysis in stainless steel flow reactors is limited by inefficient enzyme immobilization on stainless steel surfaces. Herein, we report a universal and generally applicable strategy to achieve efficient enzyme immobilization in stainless steel flow reactors by utilizing an engineered material-binding peptide (MBP) with improved binding toward stainless steel. Through this method, phytase from Yersinia mollaretii (YmPh) was immobilized and showed high activity in hydrolyzing phytic acid to produce phosphate over multiple cycles. The MBP liquid chromatography peak I (LCI) was selected and engineered for improved stainless steel binding. The variant LCISS4 (LCI A14K/Y30R/D45R) showed an 8.2-fold improved binding to stainless steel compared with the LCI wild type. YmPh-LCISS4 immobilized in additively manufactured stainless steel flow reactors exhibited strong washing resistance and high reusability. The immobilization strategy presented here, based on LCISS4, enables robust and oriented enzyme immobilization on stainless steel, making it an appealing tool for industrial biocatalysis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


