Targeted Conversion of Biomass into Primary Diamines via Carbon Shell-Confined Cobalt Nanoparticles
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
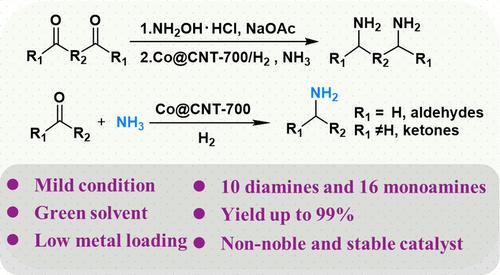
Primary diamines are valuable yet challenging to synthesize due to issues such as product and intermediate condensation and catalyst poisoning. To address these problems, effective synthesis systems must be explored. Here, 2,5-bis(aminomethyl)furan (BAMF), a biomass-derived primary diamine, is chosen as the model for constructing such a system. A series of carbon-shell confined Co nanoparticles (Co@CNT-x) are fabricated to synthesize BAMF. The Co@CNT-700 catalyst, with ca. 4 layers of carbon shells, achieves an outstanding 96% isolated yield of BAMF through the reductive amination of 2,5-diformylfuran dioxime. In this system, an excess NH3 atmosphere is necessary to prevent condensation reactions by competitive reactions, while the carbon shells protect the catalyst from NH3 and amine poisoning. Control experiments indicate that 2,5-diformylfuran dioxime primarily follows a H2-assisted dehydration pathway to form key imine intermediates, while side products such as amides and nitriles can also eventually be converted into BAMF by Co@CNT-700, leading to its excellent selectivity. Notably, by employing a sequential three-step strategy, ca. 87% BAMF can be achieved by directly using biomass as the raw material. To evaluate the tolerance of this system, 9 other important aromatic, cycloalkyl, and linear alkyl primary diamines, such as 1,4-cyclohexanediamine, are obtained in high yields of 87−99%.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


