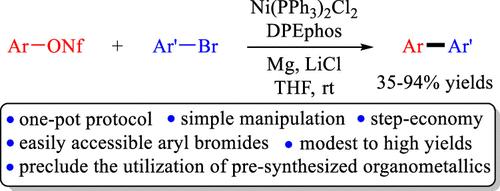
Direct Cross-Couplings of Aryl Nonaflates with Aryl Bromides under Nickel Catalysis
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The direct cross-couplings of aryl nonaflates with aryl bromides could be successfully accomplished by utilizing nickel as the catalyst, magnesium as the metal mediator, and lithium chloride as the additive. The reactions proceeded efficiently in THF at room temperature to produce the desired biaryls in moderate to good yields, showing both a reasonable substrate scope and functional group tolerance. Additionally, an equally good performance could be realized when the reaction was subjected to scale-up synthesis. Preliminary study suggested that the reaction presumably proceeds through the in situ formation of an arylmagnesium reagent as the key reaction intermediate.
镍催化下芳基溴化物与非氟化物的直接交联
以镍为催化剂,镁为金属介质,氯化锂为添加剂,可以成功地实现芳基非磺酸盐与芳基溴化物的直接交联。反应在室温下高效进行,以中等至较高的产率生产出所需的双芳基,显示出合理的底物范围和官能团耐受性。此外,当进行放大合成时,可以实现同样良好的性能。初步研究表明,该反应可能是通过原位生成芳基镁试剂作为关键反应中间体进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


