Switching Methane Selectivity in Carbon Dioxide Electroreduction via Confining Copper(I) Oxide Nanocubes by Polyimine Shells
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
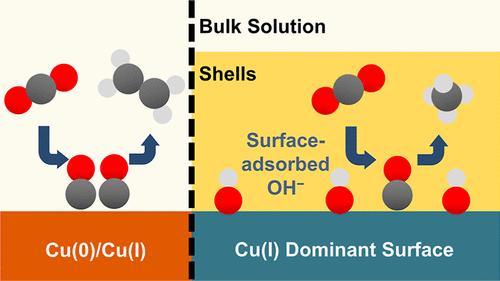
The selective formation of C1 or C2 products remains a significant challenge in electrochemical carbon dioxide reduction reaction (eCO2RR). Attaining large-scale CH4 production is significant in advancing a carbon-neutral economy. However, hydrogenation simultaneously competes with C–C coupling and hydrogen evolution reactions, which poses substantial obstacles in steering a single reaction pathway. Herein, we promote CH4 production by encapsulating Cu2O nanocubes with polyimine shells serving as diffusion barriers. The simple encapsulation with 50 nm thick shells significantly enhances the CH4-to-C2H4 ratio of Cu2O nanocatalysts from 0.14 to 8.9, achieving a Faradaic efficiency of 50.2% for CH4 at a partial current density of −201 mA cm–2 in alkaline electrolytes. In situ X-ray absorption and Raman spectroscopy demonstrate that encapsulation promotes the accumulation of surface-adsorbed hydroxide ions and significantly stabilized Cu(I) species throughout eCO2RR, with over 82% Cu(I) retention at −200 mA cm–2 for 2 h. Density functional theory calculations support the high coverage of surface-adsorbed hydroxide ions favored *CO hydrogenation over dimerization. This shell encapsulation strategy provides a straightforward approach to modulating eCO2RR mechanisms and product selectivity by tuning local chemical environments and Cu-oxidation states.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


