A Model Approach to Uncover the Role of the IrOx Crystallographic Structure and Chemistry on OER Activity and Stability via Annealing a Sacrificial Template
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
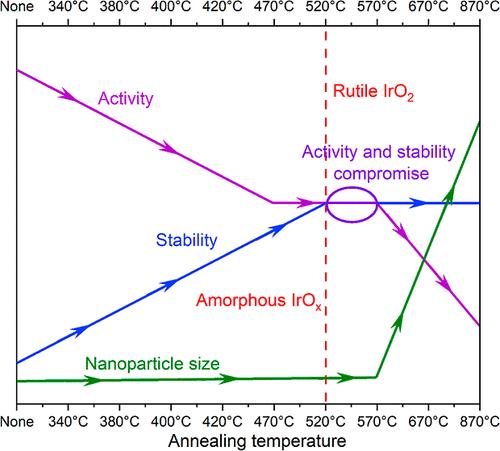
Iridium oxide nanoparticles (IrOx NPs) hold promise to lower the catalyst cost of proton-exchange membrane water electrolyzers (PEMWE). However, their enhanced oxygen evolution reaction (OER) activity often comes at the expense of stability. Achieving a delicate balance between these two conflicting properties requires a comprehensive understanding of how the structural, morphological, and chemical characteristics of IrOx NPs influence them. To address this challenge, we synthesized IrOx NPs supported on carbon (IrOx/C), and annealed them in air at temperatures ranging from 340 to 870 °C. We obtained a library of materials, ranging from small, amorphous IrOx NPs supported on carbon to larger self-supported crystalline IrO2. Using this library, we evidence the critical role of the IrOx particle size, crystallinity and chemistry on both the OER activity and catalyst stability. We further identify that an annealing temperature of 520 °C provides an optimal balance between OER activity and stability, and we demonstrate size control of unsupported small IrO2 NPs at temperatures above 500 °C, highlighting the significant role of the sacrificial support in shaping nanostructured IrO2 catalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


