A high-performance aqueous asymmetric supercapacitor based on dual redox-electrolyte enhanced systems
IF 5.8
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
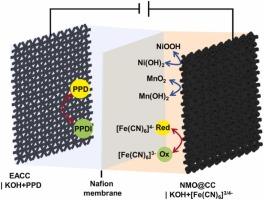
Aqueous supercapacitors (SCs) have attracted extensive attention owing to the low cost, high safety, easy handling, environment friendliness and high ionic conductivity of aqueous electrolytes. However, their energy density is constrained by the voltage threshold of water decomposition. To overcome this limitation, asymmetric SCs (ASCs) have been designed to expand the cell voltage. Moreover, the development of novel electrode materials and suitable redox-active electrolytes holds promise for achieving enhanced capacitance and increased energy density. In the present study, Ni(OH)2/Mn (hydr)oxide was loaded on activated carbon cloth via an electrochemical deposition method. It was then paired with a redox electrolyte of KOH + [Fe(CN)6]3-/4- to serve as the positive compartment for the construction of a redox electrolyte enhanced aqueous ASC. Meanwhile, electrochemically activated carbon cloth coupled with a KOH + p-phenylenediamine redox electrolyte was utilized as the negative compartment. The resulting aqueous ASC exhibited a broad cell voltage of 1.7 V and a high capacitance of 410.8 mF cm-2 at a current density of 40 mA cm-2. Its energy density reached 3.7 Wh L-1 at a power density of 396.2 W L-1, which remained 2.2 Wh L-1 even at a higher power density of 776.9 W L-1. This work provides an efficient strategy for fabricating high-performance aqueous SCs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


