Mechanism of Copper Vanadate Catalysts for Promoting CO Catalytic Oxidation Activity and SO2 Tolerance
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
A series of copper vanadates were synthesized and characterized to assess their efficacy in the low-temperature catalytic oxidation of CO. Cu3V2O8 was identified as the most active phase due to its unique electronic structure and highly reactive lattice oxygen─resulting from the solid electronic interactions between Cu and V within the Cu2+–O–V5+ asymmetric oxygen network─which offers superior catalytic performance by enhancing CO adsorption and oxidation. Additionally, the Cu3V2O8 catalyst exhibited remarkable resistance to sulfur poisoning, a characteristic attributed to its unique crystal structure and electron distribution that impedes sulfate formation. This study offers valuable insights into the design of robust, sulfur-resistant catalysts for the control of industrial CO emissions.
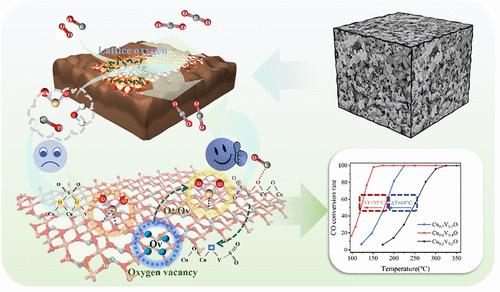
钒酸铜催化剂促进CO催化氧化活性和SO2耐受性的机理
合成了一系列钒酸铜,并对其低温催化氧化CO的效果进行了表征。Cu3V2O8被认为是最活跃的相,因为它具有独特的电子结构和高活性的晶格氧(由Cu2+ -O-V5 +不对称氧网络中Cu和V之间的固体电子相互作用引起),通过增强CO的吸附和氧化而具有优异的催化性能。此外,由于其独特的晶体结构和电子分布阻碍了硫酸盐的形成,Cu3V2O8催化剂表现出了显著的抗硫性。这项研究提供了有价值的见解,为设计稳健,抗硫催化剂控制工业CO排放。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


