Zinc Acetate/Ionic Liquid Hybrid Catalysts for the Synthesis of Dimethyl Carbonate Through Urea Methanolysis: Kinetics, Molecular Dynamic Simulation, and Mechanism Clarification
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
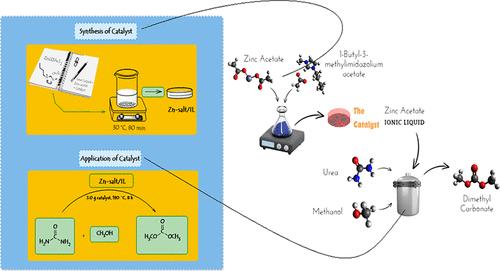
In this study, 1-butyl-3-methylimidazolium acetate was synthesized and then mixed with zinc acetate as a novel hybrid catalyst for the synthesis of dimethyl carbonate (DMC) through urea methanolysis. The Fourier transform infrared analysis (FT-IR) showed that a connection formed between the cation part of the ionic liquid (IL) and zinc acetate, confirming the successful synthesis of the zinc acetate/IL catalyst. The effects of the urea-to-methanol molar ratio and different weight ratios of ILs to Zn-based salt on the DMC yield were studied using an equipped batch catalyst-test setup. Generally, complete urea conversion was achieved under the optimal operating conditions, i.e., a 1:36.6 molar ratio of urea/methanol, 3.0 g of catalyst, 190 °C, and 8 h of reaction time. 1-Butyl-3-methylimidazolium acetate-promoted zinc acetate with a weight ratio of 1:2 indicated a superior DMC yield (∼15%). Reaction kinetic results disclosed that 1-butyl-3-methylimidazolium acetate has a strong promoting effect on the Zn-based catalyst for DMC production, especially at the early times of the reaction. The recyclability tests showed that zinc, the hybrid catalyst, remains stable and efficient even after four recovery/reuse runs. The hydrogen bonding between the C2–H in the cation of IL and hydrogen bond acceptor groups in zinc acetate plays a significant role in promoting the reaction. Molecular dynamics (MD) simulations were applied to investigate energy parameters, including potential, kinetics, interactions, and bonding energies, in systems involving methanol and urea, with Zn-based salts and ILs. There is an agreement between simulation and experimental results, showing that the zinc acetate/ionic liquid catalyst can create the most effective catalytic environment for urea methanolysis, enhancing DMC production. From an industrial viewpoint, equipment size and expenditures can be favorably reduced by promoting the reaction kinetics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


