The cyclized polyacrylonitrile coating current collector for directional deposition of lithium metal induced by Zn nanosheets
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Lithium (Li) with high theoretical specific capacity and being the lightest metal, so Li metal anodes has attracted significant interest all over the world. This study introduces the first application of cyclized polyacrylonitrile (CPAN) coatings on commercial copper foil to modulate the nucleation and growth of Zn, resulting in the development of Cu@CPAN@Zn (CCZ) composite current collectors for Li metal anodes. The phase changes during the CCZ preparation process are investigated, along with the mechanisms by which CPAN influences Zn deposition behavior. Li deposition tests demonstrate that the ionic conductivity and mechanical properties of CPAN facilitate Li deposition within the CPAN layer, while the incorporation of Zn induces the uniform deposition of Li and suppresses Li dendrite growth. Electrochemical evaluations indicate that the CCZ current collector exhibits a lower Li deposition potential and extended cycling life. Notably, the CCZ@Li symmetric cell shows stable cycling for 1200 h at 0.5 mA cm-2 and 0.5 mAh cm-2. These results underscore the promising potential of CCZ composite current collectors for improving the performance of Li metal batteries.

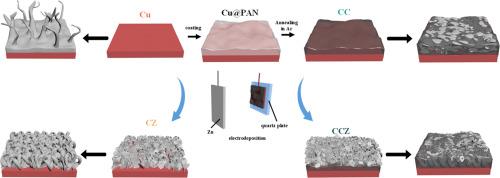
用于锌纳米片诱导金属锂定向沉积的环化聚丙烯腈涂层集流剂
锂金属阳极具有较高的理论比容量和最轻的金属性质,引起了世界各国的极大兴趣。本研究首次在商用铜箔上应用环化聚丙烯腈(CPAN)涂层来调节Zn的成核和生长,从而开发出Cu@CPAN@Zn (CCZ)复合集流器,用于锂金属阳极。研究了CCZ制备过程中的相变,以及CPAN影响Zn沉积行为的机理。Li沉积实验表明,CPAN的离子电导率和力学性能有利于Li在CPAN层内的沉积,而Zn的掺入诱导Li均匀沉积,抑制Li枝晶的生长。电化学评价表明,CCZ集流器具有较低的锂沉积电位和较长的循环寿命。值得注意的是,CCZ@Li对称电池在0.5 mA cm-2和0.5 mAh cm-2下可稳定循环1200小时。这些结果强调了CCZ复合集流器在提高锂金属电池性能方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


