Electrolytic cement clinker precursor production sustained through orthogonalization of ion vectors
IF 32.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
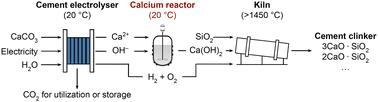
Electrochemical reactors can reduce the carbon intensity of cement production by using electricity to convert limestone (CaCO3) into Ca(OH)2, which can be converted into cement clinker by reacting with silica (SiO2) at high temperatures. A key challenge with electrochemical reactors is that the deposition of solid Ca(OH)2 at the membrane leads to unacceptably low energy efficiencies. To address this challenge, we connected the electrochemical reactor used for limestone calcination (“cement electrolyser”) to a distinctive chemical reactor (“calcium reactor”) so that Ca(OH)2 forms in the calcium reactor instead of within the electrochemical reactor. In this tandem system, the cement electrolyser generates H+ and OH− in the respective chemical and cathode compartments. The H+ then reacts with CaCO3 to release Ca2+, which is diverted into the calcium reactor to react with the OH− to form Ca(OH)2. We fabricated a composite membrane to selectively block the transport of Ca2+ into the cathode compartment. Charge balance in the cement electrolyser was enabled with monovalent ions (e.g., K+) as the positive charge carrier. This orthogonalized ion management was validated by operando imaging. The tandem reactor enabled the electrolysis process to operate for 50 hours at 100 mA cm−2 without any voltage increase, which represents a meaningful step forward for electrochemical cement clinker precursor production.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy & Environmental Science
化学-工程:化工
CiteScore
50.50
自引率
2.20%
发文量
349
审稿时长
2.2 months
期刊介绍:
Energy & Environmental Science, a peer-reviewed scientific journal, publishes original research and review articles covering interdisciplinary topics in the (bio)chemical and (bio)physical sciences, as well as chemical engineering disciplines. Published monthly by the Royal Society of Chemistry (RSC), a not-for-profit publisher, Energy & Environmental Science is recognized as a leading journal. It boasts an impressive impact factor of 8.500 as of 2009, ranking 8th among 140 journals in the category "Chemistry, Multidisciplinary," second among 71 journals in "Energy & Fuels," second among 128 journals in "Engineering, Chemical," and first among 181 scientific journals in "Environmental Sciences."
Energy & Environmental Science publishes various types of articles, including Research Papers (original scientific work), Review Articles, Perspectives, and Minireviews (feature review-type articles of broad interest), Communications (original scientific work of an urgent nature), Opinions (personal, often speculative viewpoints or hypotheses on current topics), and Analysis Articles (in-depth examination of energy-related issues).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


