Rational Design, Synthesis, and Biological Evaluation of Novel c-Met Degraders for Lung Cancer Therapy
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
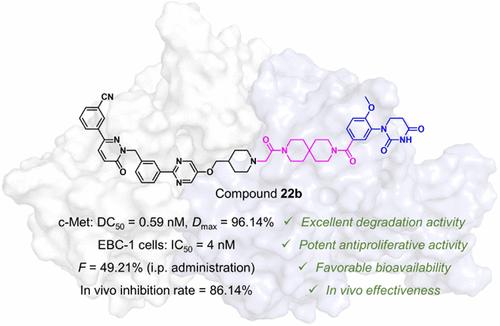
Cellular-mesenchymal epithelial transition factor (c-Met) is an attractive target for treating multiple cancers. Despite plentiful c-Met inhibitors have been developed, some issues, including the acquired drug resistance to c-Met inhibitors, have emerged to hamper their application in clinical treatment. Degradation of c-Met offers an opportunity to solve these issues. In this study, we developed a series of c-Met degraders, and the optimal compound 22b can efficiently degrade c-Met with a DC50 value of 0.59 nM in EBC-1 cells. Mechanistic studies revealed that compound 22b induced c-Met degradation via proteasome-mediated pathway. In addition, compound 22b suppressed the proliferation and also induced apoptosis of EBC-1 cells, outperforming the corresponding inhibitor tepotinib. Importantly, compound 22b showed favorable pharmacokinetic properties and significantly induced tumor regression in a xenograft model without obvious toxicity. In brief, this study provided compound 22b as a novel c-Met degrader for lung cancer therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


